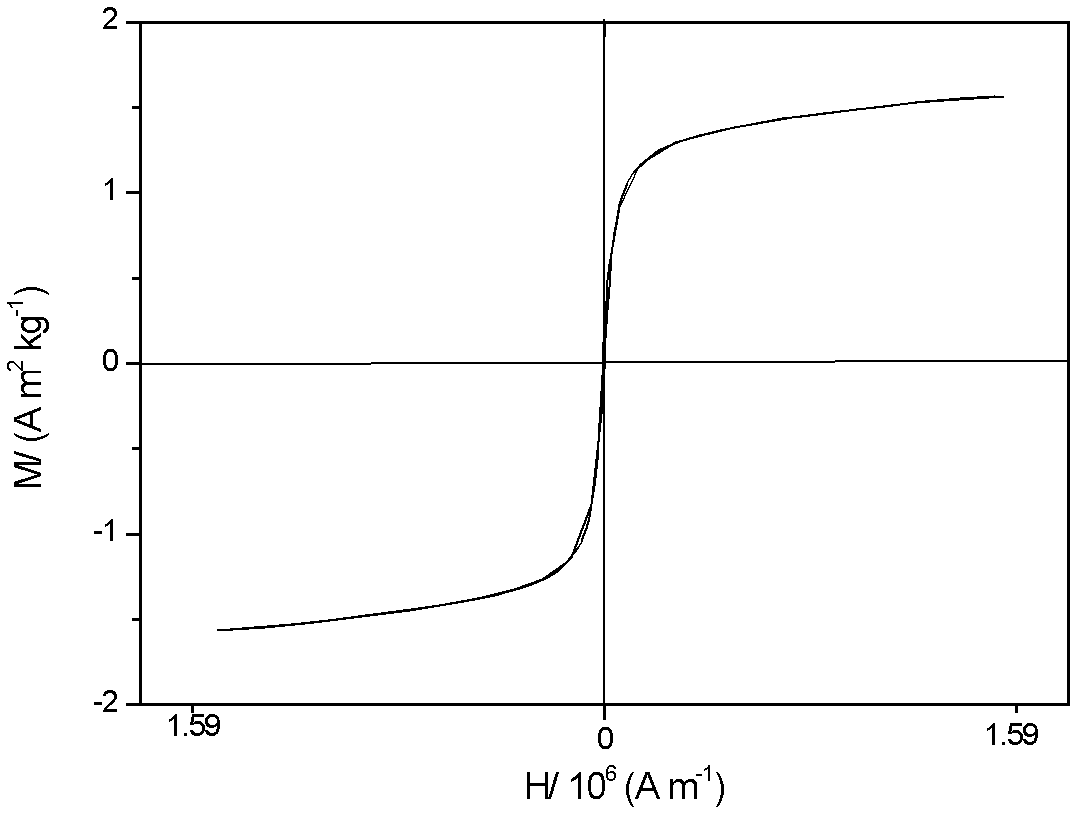
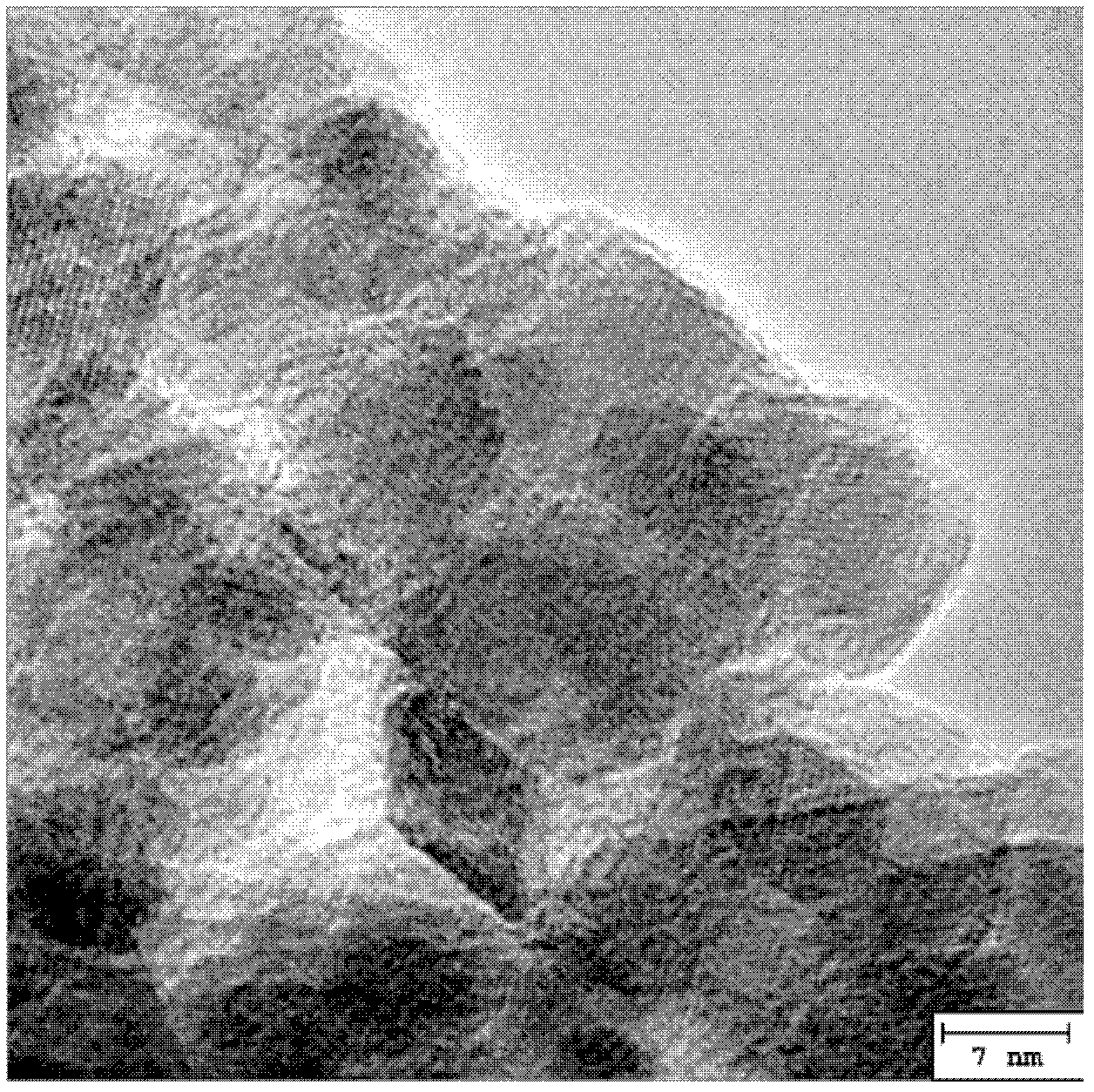Magnetic bone cement material and method for preparing magnetic bone cement
A bone cement and magnetic technology, applied in medical science, prosthesis and other directions, can solve the problems of short curing time of bone cement, poor clinical operation effect, unfavorable minimally invasive treatment, etc., and achieve extended curing time and good magnetic heat generation performance. , The effect of meeting the needs of clinical use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
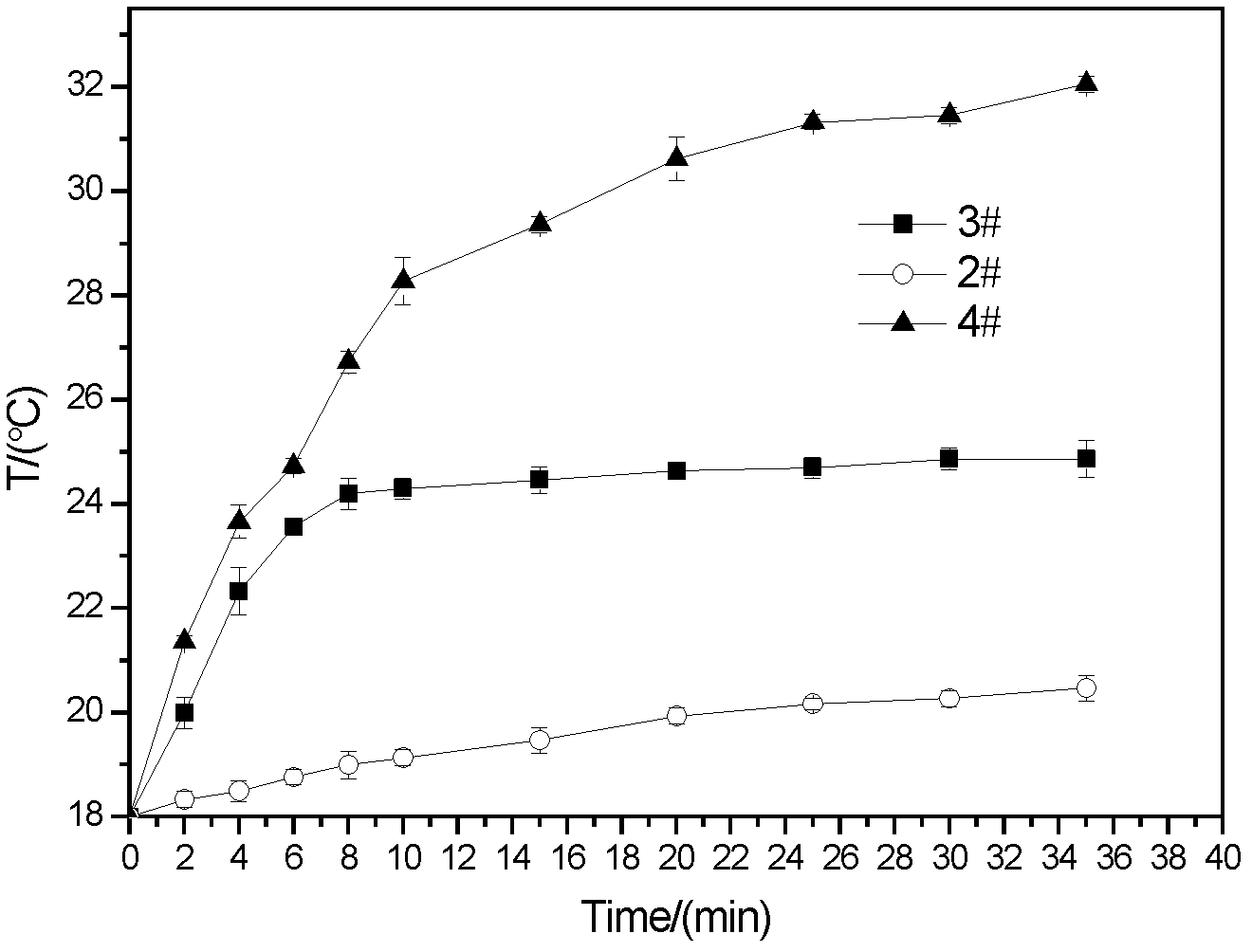
[0021] Embodiment 1 (2#)
[0022] The magnetic bone cement material of this embodiment is composed of the following components by weight: 100 parts of a mixture of β-calcium phosphate and calcium dihydrogen phosphate monohydrate with a molar ratio of 1:1, and 5 parts of nanometer ferric oxide.
[0023] Wherein the β-calcium phosphate is prepared by the following method: take calcium carbonate powder and add water to reconcile into a slurry, and according to the Ca / P molar ratio of 1.49:1, add the slurry to a 1.42mol / L phosphoric acid solution under ultrasonic vibration and vigorous stirring , after adding, continue to ultrasonically fully react, filter the precipitate, wash and dry; calcinate the precipitate at 1100°C for 2 hours, the heating rate is 5°C / min, cool after calcining, and ball mill until it passes through a 200-mesh sieve.
[0024] Wherein nano ferric oxide is prepared by the following method: take FeSO 4 ·7H 2 O and FeCl 3 ·6H 2 O prepare ferrous salt and fer...
Embodiment 2
[0029] Embodiment 2 (3#)
[0030] The magnetic bone cement material and magnetic bone cement of the present embodiment are basically the same as those of Example 1, the difference being that the addition amount of nano-ferric oxide is 10 parts, and the specific embodiment content is: the magnetic bone cement material of the present embodiment, The composition is composed of the following components in parts by weight: 100 parts of a mixture of beta calcium phosphate and calcium dihydrogen phosphate monohydrate with a molar ratio of 1:1, and 10 parts of nano ferric oxide.
[0031] Wherein the β-calcium phosphate is prepared by the following method: take calcium carbonate powder and add water to reconcile into a slurry, according to the Ca / P molar ratio of 1.50:1, add the slurry to a 1.43mol / L phosphoric acid solution under ultrasonic vibration and vigorous stirring , after adding, continue to ultrasonically fully react, filter the precipitate, wash and dry; calcinate the precip...
Embodiment 3
[0037] Embodiment 3 (4#)
[0038] The magnetic bone cement material and the magnetic bone cement of this embodiment are basically the same as those of Example 1, the difference being that the addition amount of nano ferric oxide is 20 parts, and the specific embodiment content is as follows: the magnetic bone cement material of this embodiment, It is composed of the following components in parts by weight: 100 parts of a mixture of beta calcium phosphate and calcium dihydrogen phosphate monohydrate with a molar ratio of 1:1, and 20 parts of nano ferric oxide.
[0039] Wherein the β-calcium phosphate is prepared by the following method: take calcium carbonate powder and add water to reconcile into a slurry, and according to the Ca / P molar ratio of 1.52:1, add the slurry to a 1.45mol / L phosphoric acid solution under ultrasonic vibration and vigorous stirring , after adding, continue to ultrasonically fully react, filter the precipitate, wash and dry; calcinate the precipitate at 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Compressive strength | aaaaa | aaaaa |
| Compressive strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com