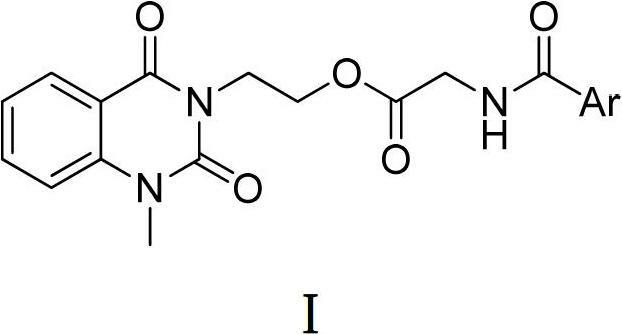
3-substituted-1-methyl-quinazoline-2,4-dione compounds, preparation method and application thereof
A technology of quinazolinedione and compound, which is applied in the field of preparation of the compound, can solve the problems such as reduction and loss of therapeutic effect, increase of bacterial drug resistance, etc., achieves the solution of bacterial drug resistance, simple preparation method, and easy-to-obtain raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1, the preparation of 1-methyl-2 shown in general formula I, 4 (1H, 3H)-quinazoline diketones
[0024] 1, the preparation of intermediate 2 (methyl o-thaminobenzoate)
[0025]
[0026] In a 250ml flask with a drying tube, add 16g of raw material 1 (methyl anthranilate) and 80ml of dimethylformamide (DMF), then dropwise add a mixture of 15.6g of iodomethane and 20ml of DMF, and add to room temperature React for 1 hour, then heat to 50°C and react for 7 hours. After the reaction, cool to room temperature, add 200ml of water, extract with ethyl acetate (3×60ml), combine the ethyl acetate extracts, dry over anhydrous magnesium sulfate, and filter with suction , the filtrate was rotary evaporated to remove the solvent, and 11.646 g of intermediate 2 (yellow liquid) was obtained, with a yield of 66.7%.
[0027] 2, the preparation of intermediate 3 (o-methylaminobenzoic acid)
[0028]
[0029] In a 250ml flask, add 29.242g of intermediates, 40ml of water, 30...
Embodiment 2
[0066] The antimicrobial activity of 1-methyl-2 shown in embodiment 2, general formula I, 4 (1H, 3H)-quinazoline diketones
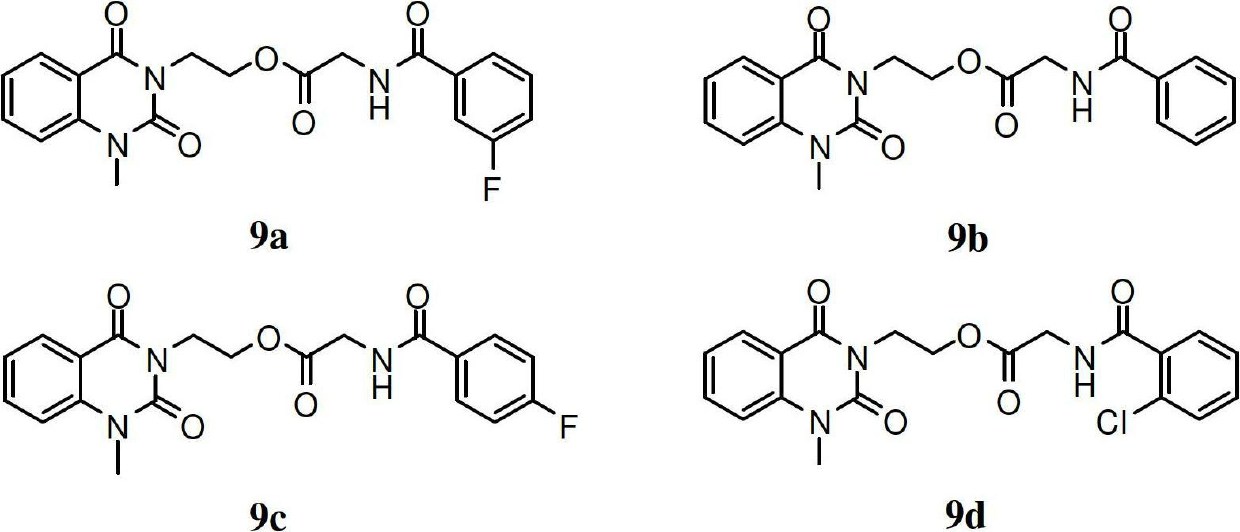
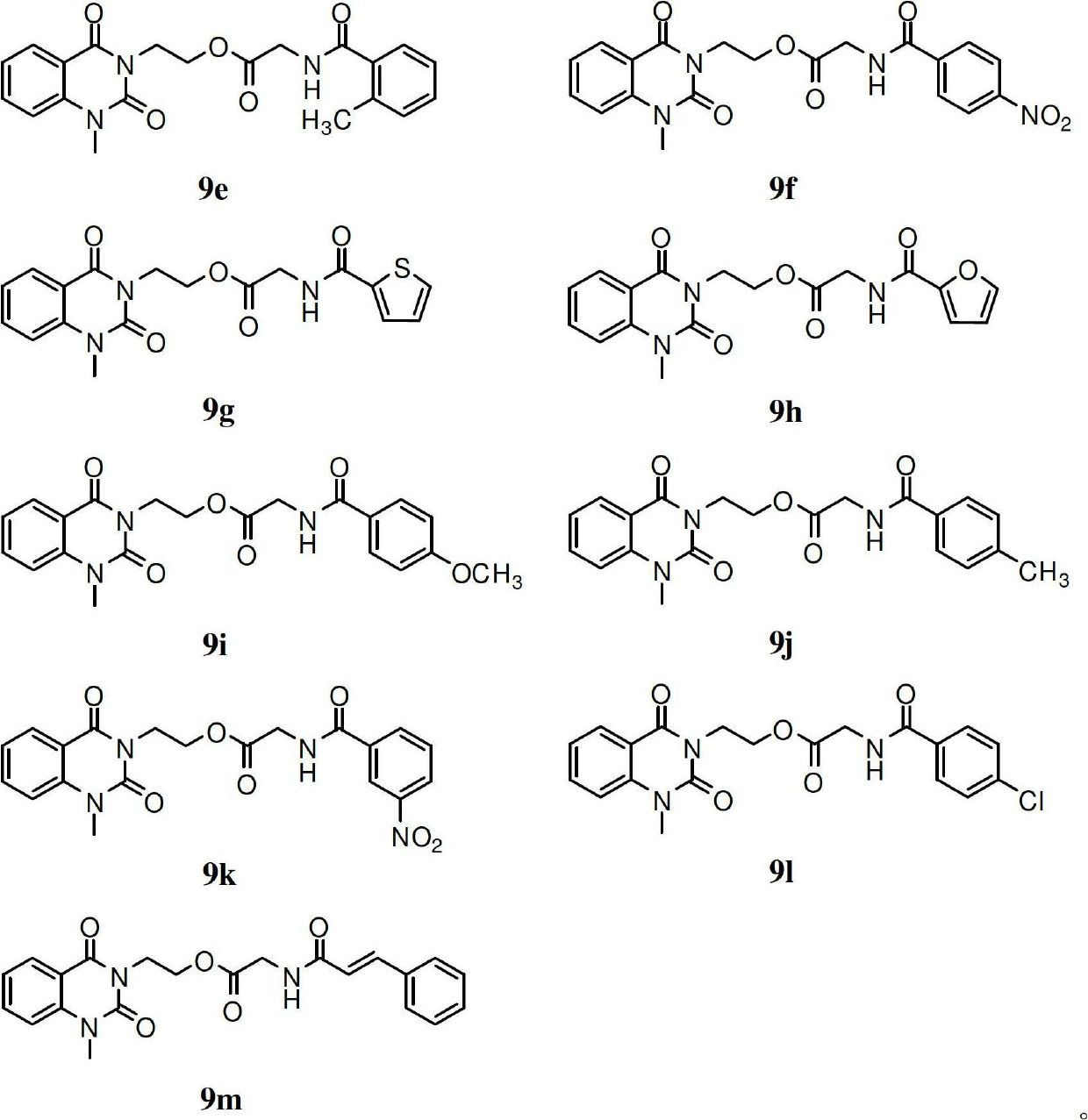
[0067] After dissolving the compounds 9a-m prepared in Example 1 with a small amount of dimethyl sulfoxide, they were diluted with sterile water to make a solution of a certain concentration, and then diluted with water to prepare a series of sample solutions with gradient concentrations. Set streptomycin sulfate and polyoxin D as positive controls; pour the sterilized agar solid medium into the plate, and after cooling, spread 50 μl of the bacterial solution evenly on the surface of the agar, and then apply the sample solution with different concentrations The soaked filter paper pieces were pasted on the surface of the agar, cultured at 37°C for 24 hours (bacteria) or 25°C for 48 hours (fungus), and the minimum inhibitory concentration (MIC) was determined. The results are shown in Table 1
[0068] Antimicrobial activity (MIC, μg / ml) of table 13-subst...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com