Cracking connecting unit for tetrahydrofuran ether derivatives and application of cracking connecting unit
A technology of tetrahydrofuran ether and linking units, which is applied in the fields of chemical synthesis and biochemistry, can solve problems such as insufficient mild cracking conditions, low cracking efficiency, and short read length, and achieve high-efficiency cracking effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
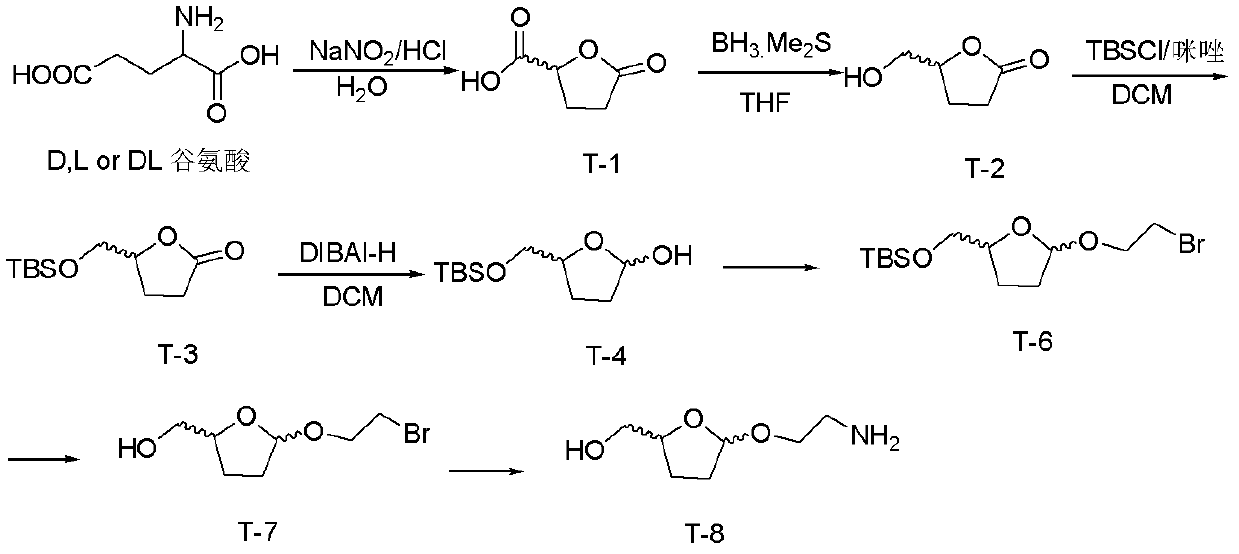
[0063] Embodiment 1, when n=0, the synthesis of connecting unit T-8
[0064] The schematic diagram of the synthetic route of linking unit T-8 (5-hydroxymethyl-2-(2-aminoethoxy)-tetrahydrofuran) is as follows figure 1 As shown, the synthesis steps are as follows:
[0065] (1) Synthesis of T-1: D, L or DL-glutamic acid is reacted in the presence of sodium nitrite to obtain T-1.
[0066] The steps are as follows: add 10 g (68 mmol) of D, L or DL-glutamic acid into a 500 mL single-necked bottle, add hydrochloric acid (14 mL of concentrated hydrochloric acid dissolved in 28 mL of water), and dissolve the solid. Stir in an ice bath for 30 min, then continue to add a solution of sodium nitrite (7 g, 100 mmol, dissolved in 30 mL of water) dropwise in an ice bath, during which reddish-brown smoke is produced. After the dropwise addition, the reaction was continued for 3 h in an ice bath, and then moved to room temperature overnight. The water was distilled off under reduced pressu...
Embodiment 2
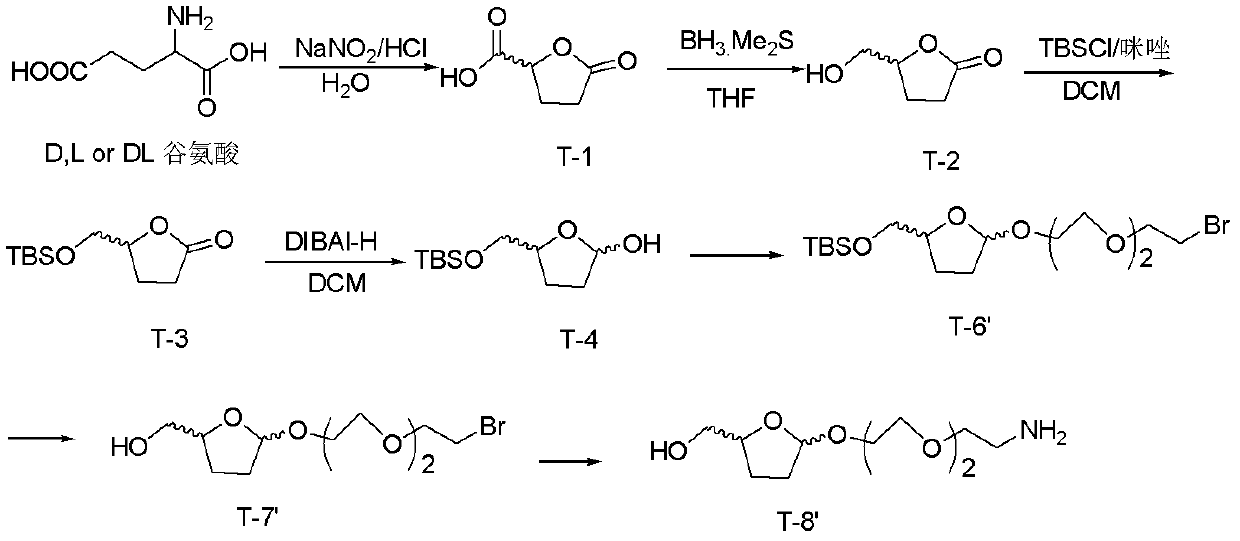
[0088] Embodiment 2, when n=2, the synthesis of connecting unit T-8'
[0089] The schematic diagram of the synthetic route is as figure 2 As shown, the synthesis steps are as follows:
[0090] (1) The synthesis of T-1 to T-4 is the same as that described in Example 1.
[0091] (2) Synthesis of T-6': T-4 reacts with bromotriethylene glycol to obtain T-6'.
[0092] The specific steps are: take 232mg T-4 (1mmol), add 15mL dichloromethane to dissolve, add 426mg monobromotriethylene glycol (2mmol), add 50mg Amberlyst A-15, add reflux at 50°C for 2h. Stop the reaction, remove A-15 by filtration, and wash with saturated sodium bicarbonate and saturated brine. The solvent was distilled off under reduced pressure to obtain 600 mg of light yellow oily liquid. Packed with silica gel column, petroleum ether: ethyl acetate = 5: 1 column separation to obtain 128 mg of light green T-6' pure product with a yield of 30%. Cis: 1 H NMR (CDCl 3 , 400M): δ5.17(1H, d, J=4.8Hz), 4.17~4.08(...
Embodiment 3
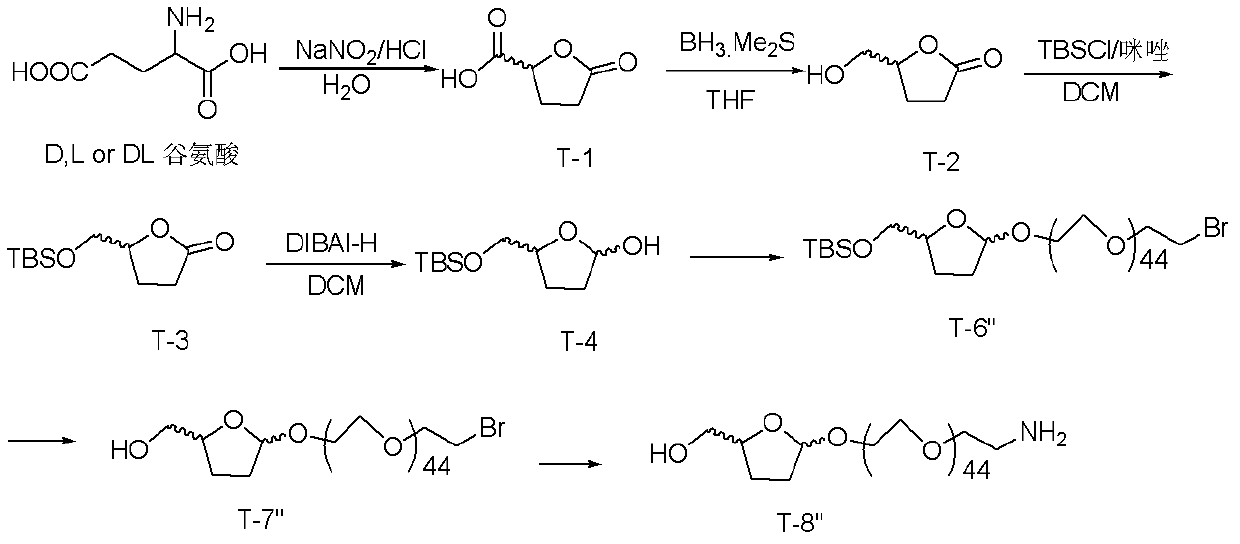
[0098] Embodiment 3, when n=44, the synthesis of linking unit T-8 "
[0099] The schematic diagram of the synthetic route is as image 3 As shown, the synthesis steps are as follows:
[0100] (1) The synthesis of T-1 to T-4 is the same as that described in Example 1.
[0101] (2) Synthesis of T-6 ": T-4 reacts with brominated polyethylene glycol 2000 to obtain T-6".
[0102] The steps are as follows: take 1 mmol of T-4, add 15 mL of dichloromethane to dissolve, add 2 mmol of monobrominated polyethylene glycol 2000, add 50 mg of Amberlyst A-15, add reflux at 50°C for 2 hours. Stop the reaction, remove A-15 by filtration, and wash with saturated sodium bicarbonate and saturated brine. The solvent was distilled off under reduced pressure to obtain 600 mg of light yellow oily liquid. Packed on a silica gel column and separated with petroleum ether: ethyl acetate = 5:1 column to obtain 213 mg of light green T-6" pure product. 1 H NMR (CDCl 3 , 400M): δ5.19(1H, d, J=4.8Hz), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com