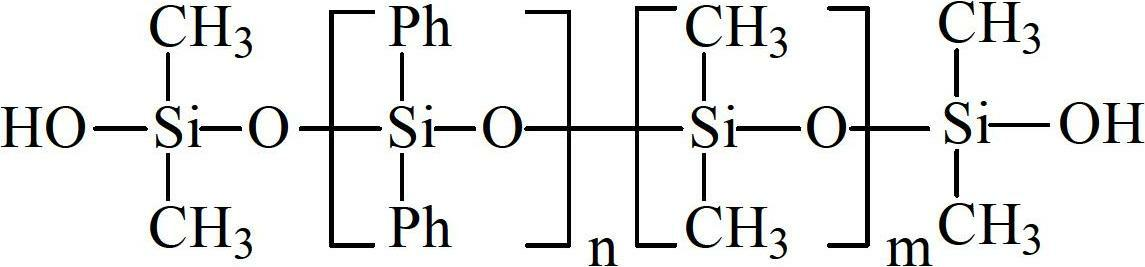Alpha, omega-hydroxyl-terminated diphenyl siloxane and dimethhyl siloxane copolymer and preparation method thereof
A technology of dimethylsiloxane and diphenylsiloxane, which is applied in the field of organic silicon materials, can solve the problems of complex preparation process, complex process, easy breakage and the like of a mixed ring body, and achieves improved storage stability and simple process. , less corrosive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]Preparation of hydroxyl-terminated diphenylsiloxane-dimethylsiloxane copolymer
[0040] Add 45g of octaphenylcyclotetrasiloxane (D 4 Ph2 ) and 300g of dimethylsiloxane ring body (DMC), then add 0.2g of potassium silanolate catalyst and 1.5g of dimethyl sulfoxide. After closing the reaction system, start stirring, and raise the temperature to 150°C. After 20 minutes, the solid powder in the reaction bottle almost disappeared. Continue the reaction for 40 minutes. Add 4.0 g of deionized water to the reaction system for degradation reaction, and continue stirring for 2 hours.
[0041] After the degradation reaction, add 0.3g of propionic acid, neutralize the reaction for 1 hour, heat up to 160°C and vacuumize to 1mmHg for 4 hours to remove low molecular weight, and obtain a colorless, transparent, odorless hydroxyl-terminated diphenylsiloxane after cooling Alkane-dimethylsiloxane copolymer products. The viscosity of gained product is 4500mPas, and volatile matter content...
Embodiment 2
[0043] As described in Example 1, the difference is:
[0044] Add 130 g of D to the reaction vial 4 Ph2 And 300g of DMC, 0.3g of potassium silanolate catalyst and 2.5g of N,N-methylacetamide. Close the reaction system and raise the temperature to 150°C. After about 30 minutes, the solid powder in the reaction bottle almost completely disappears, and the reaction is continued for 50 minutes. The rest is the same as in Example 1, and a colorless, transparent, and odorless hydroxyl-terminated diphenylsiloxane is obtained. -Dimethicone copolymer products.
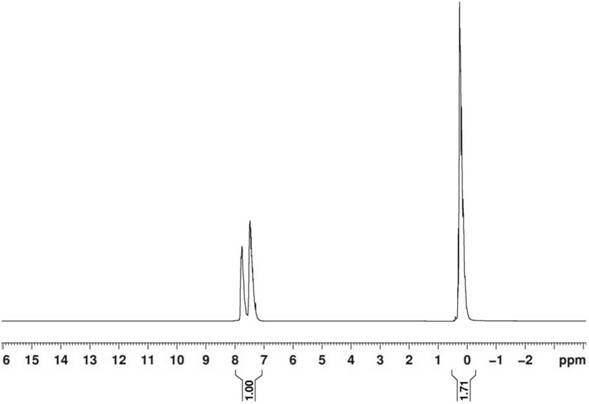
[0045] The viscosity, volatile content, refractive index, phenyl chain segment content and surface vulcanization time of the product were tested respectively, and the results are shown in Table 1.
Embodiment 3
[0047] As described in Example 1, the difference is:
[0048] Add 270 g of D to the reaction vial 4 Ph2 And the DMC of 300g, potassium siliconate catalyst 0.3g and tri-n-butyl phosphorus oxide 5.5g. Close the reaction system and raise the temperature to 150°C. After about 40 minutes, almost all the solid powder in the reaction bottle disappears, and the reaction is continued for 50 minutes. The rest is the same as in Example 1, and a colorless, transparent, odorless hydroxyl-terminated diphenylsiloxane is obtained. -Dimethicone copolymer products.
[0049] The viscosity, volatile content, refractive index, phenyl chain segment content and surface vulcanization time of the product were tested respectively, and the results are shown in Table 1.
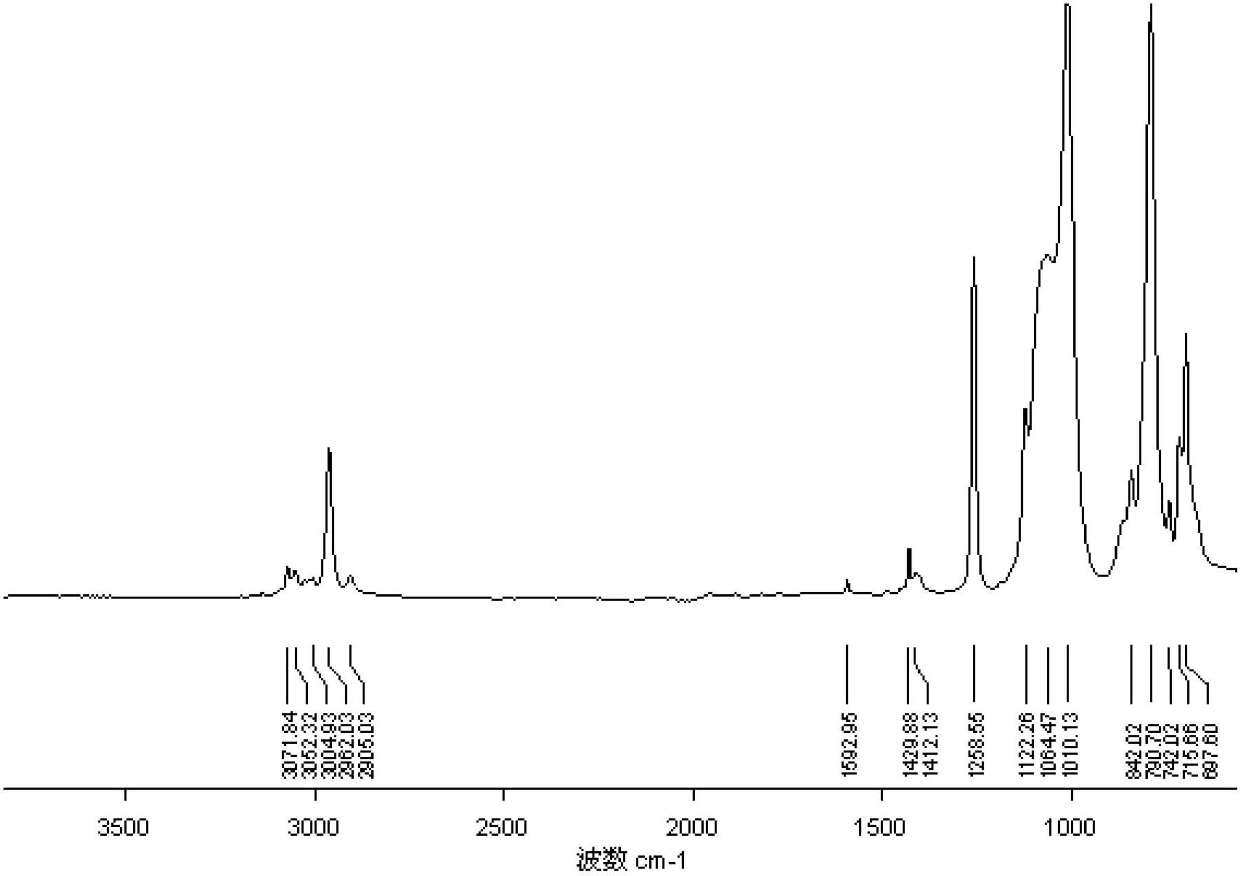
[0050] from figure 1 As can be seen in , 3071cm -1 、3052cm -1 、3004cm -1 Both are absorption peaks of hydrogen on the benzene ring, 1590cm -1 and 1120cm -1 It is the absorption peak of silicon phenyl group, 1260cm -1 It is the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com