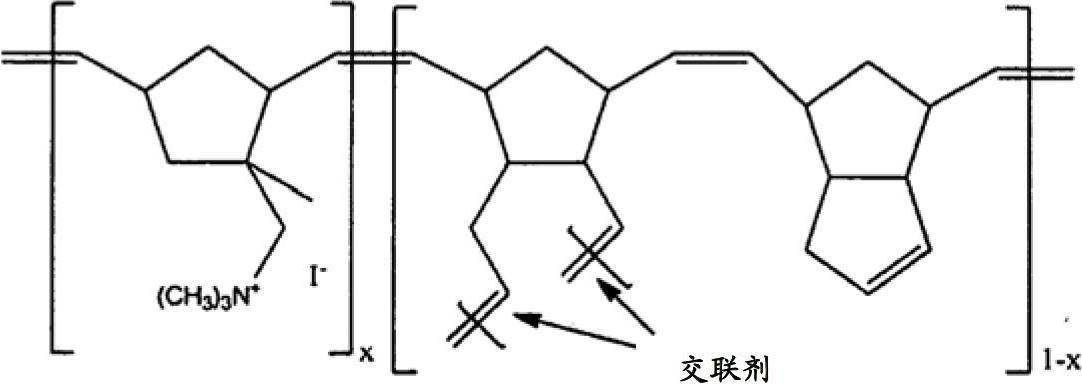
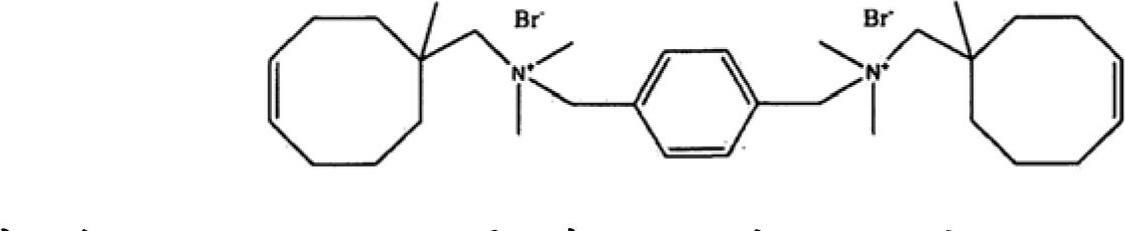
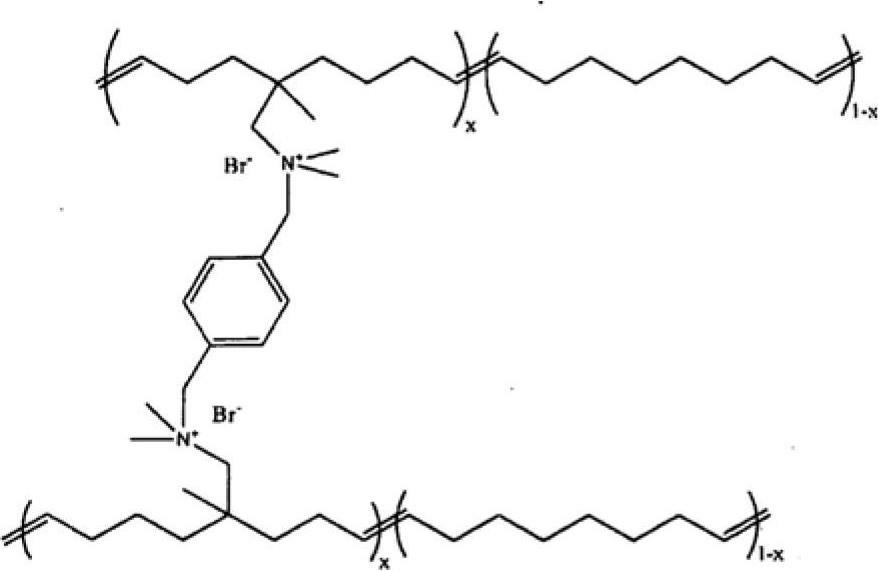
Norbornene-type polymers having quaternary ammonium functionality
A norbornene, polymer technology, applied in AAEM and, (AAEM) and including the first electrode field, can solve the problem of no anion exchange membrane
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment M1
[0064] Example M1: Synthesis of endo-5-methyl-exo-5-carboxylic acid-2-norbornene
[0065] Freshly cracked cyclopentadiene (939 g, 14.2 mol) and methacrylic acid (1203 g, 14.2 mol) were added to an appropriately sized vessel containing a magnetic stirrer. The contents were stirred for 24 hours and then left without stirring for 60 hours, during which time a white powder was observed to precipitate out of solution. To facilitate precipitation, the flask was cooled at 10°C for several hours. The precipitate was collected by vacuum filtration and washed with cold pentane (2 L, -10 °C) to remove any unreacted starting material and possible formation of NB(exo-Me)(endo-CO 2 H) By-products. The precipitated white powder (700 g) was recrystallized in hexane (~50 wt%) to give NB(endo-Me)(exo-CO 2 H) Clear crystals (621 g, 29%).
[0066] use 1 HNMR and 13 CNMR characterizes the monomer. Name this NMR signal using the numbering system shown in the figure below.
[0067]
[006...
Embodiment M2
[0069] Example M2——synthesis of internal-5-methyl-external-5-methylhydroxyl-2-norbornene
[0070] NB(inner-Me)(outer-CO 2 H) (174.8 g, 1.15 mmol) and anhydrous toluene (1000 mL) were added to an appropriately sized vessel kept under nitrogen. The vessel was equipped with a magnetic stir bar, thermometer, addition funnel and condenser. Will The premix (500 g, 70 wt% in toluene, 1.73 mol) was added to the addition funnel (brief exposure to air is acceptable if At the same time, keep the temperature of the tank at 5-20°C. After the addition was complete, the contents were heated to 100°C for 6 hours (until thin layer chromatography (TLC) showed complete reaction). The contents were cooled overnight. This solution was slowly added to a beaker containing vigorously stirred 5N HCl (1000 mL). The temperature was maintained at 4 Dry, then filter and remove the solvent under reduced pressure to give NB(inner-Me)(exo-CH 2 OH) (152g, 96%, >95% purity).
[0071] use 1 HNMR and ...
Embodiment M3
[0074] Example M3——synthesis of endo-5-methyl-exo-5-methoxyl-mesylate-2-norbornene
[0075] NB(inner-Me)(outer-CH 2 OH) (74.8 g, 540 mmol) was dissolved in 250 mL of dichloromethane in an appropriately sized vessel. Methanesulfonyl chloride (MsCl) (65.6 g, 0.54 mol) was added. The mixture was then cooled to -12.5°C with a methanol-ice bath. Triethylamine (65.6 g, 650 mmol) was slowly added dropwise while keeping the reaction temperature below -1.0 °C. A large amount of white solid precipitated out. Addition was complete after 30 minutes. The reaction was warmed to 14°C over 40 minutes. GC analysis showed that all starting material was consumed. The mixture was treated with 200 ml of water and the phases were separated. The organic phase was washed with 200 ml of 1N HCl, then brine until the wash gave pH~6. The organic portion was dried over anhydrous magnesium sulfate, filtered and rotary evaporated to afford 100 g (90% yield) of NB(endo-Me)(exo-CH 2 OMs), a light ora...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com