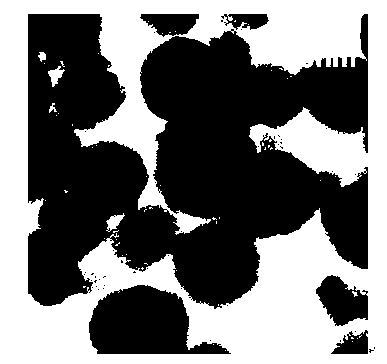Controllable preparation method of silver phosphate nanocrystal photocatalytic material
A technology of nanocrystals and silver phosphate, applied in the field of photocatalytic materials, to achieve the effect of improving photocatalytic activity, simple method, and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Take 30 mL of 1 mmol / L silver nitrate aqueous solution, then add 1 mL of 1 mmol / L sodium phytate solution, heat to boiling, keep for 3 minutes, then cool to room temperature, then add 1 mL of 0.01mol / L trisodium phosphate aqueous solution , fully stirred for 1 minute to form silver phosphate colloidal liquid, left to stand for 6 hours and then centrifuged, washed 4 times with deionized water to remove phytic acid adsorbed on the surface, and finally dried in a vacuum oven at 80 ℃ for 6 hours. The silver phosphate nanocrystals are prepared, and the average particle diameter of the obtained silver phosphate nanocrystals is 45nm.
Embodiment 2
[0021] Take 30 mL of 1 mmol / L silver nitrate aqueous solution, then add 1 mL of 1 mmol / L sodium phytate solution, heat to boiling, keep for 10 minutes, then cool to room temperature, then add 2 mL of 0.01mol / L dipotassium hydrogen phosphate Aqueous solution, fully stirred for 30 minutes to form silver phosphate colloidal liquid, left to continue to react for 6 hours, then centrifuged, washed with deionized water 4 times to remove phytic acid adsorbed on the surface, and finally dried in a vacuum oven at 80 ℃ for 6 hours The sample was obtained, and the average particle size of the obtained nano-silver phosphate crystals was 50nm.
Embodiment 3
[0023] (1) Preparation of sodium phytate solution: Dissolve 1 mmol / L of phytic acid in 1 L of water, and then add 1 mol / L of sodium hydroxide solution with a pipette until the pH of the mixed solution is 7, that is, 1 mmol / L L of sodium phytate solution;
[0024] (2) Take 30 mL of 1 mmol / L silver nitrate aqueous solution, then add 1 mL of 1 mmol / L sodium phytate solution prepared in step (1), heat to boiling, keep for 10 minutes, cool to room temperature, and then add 0.5 mL 0.01mol / L tripotassium phosphate aqueous solution, fully stirred for 1 minute to form a silver-yellow colloidal liquid of phosphate, left to stand for 6 hours and then centrifuged, and washed 4 times with deionized water to remove phytic acid adsorbed on the surface, and finally in The sample was dried in a vacuum oven at 80°C for 6 hours, and the average particle size of the obtained nano-silver phosphate crystals was 40nm.
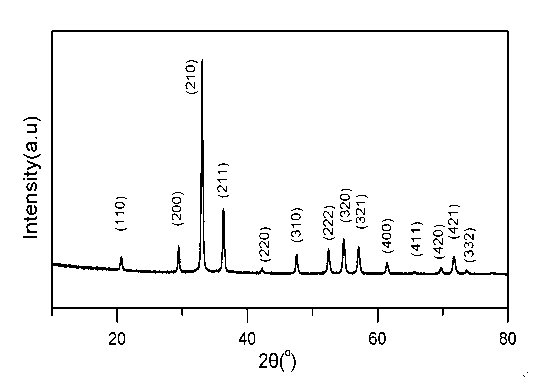
[0025] The silver phosphate nanocrystal photocatalytic materials prepared in th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com