Preparation method of transition metal nano-boride
A nano-boride, transition metal technology, applied in the direction of metal borides, boron/borides, nanotechnology, etc., can solve the problem of inability to obtain crystalline or amorphous particles with nanostructures, and achieve high practical value, The effect of reducing process conditions and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
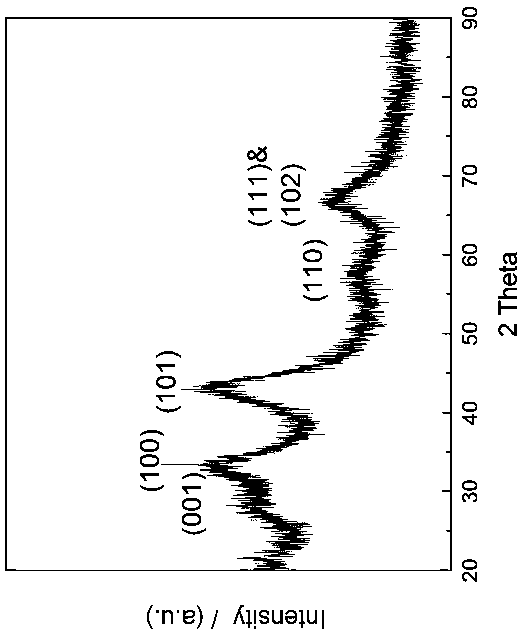
[0029] Preparation of nanocrystalline NbB 2 .
[0030] The raw material used is: anhydrous NbCl 5 (purity 98%), LiBH 4 (purity 98%), LiH (purity 95%), anhydrous LiCl (purity greater than 99%), tetrahydrofuran (purity greater than 99%), metal Na (purity greater than 98%).
[0031] Molar ratio NbCl 5 : LiBH 4 : LiH = 1 : 2 : 3, weigh NbCl in glove box 5 , LiBH 4 and LiH. Weigh anhydrous LiCl, whose mass is 8 times the total mass of the above three samples. NbCl to be weighed 5 Grind evenly with anhydrous LiCl, then weigh the LiBH 4 and LiH into NbCl 5 And LiCl mixture, further grinding evenly. The above uniformly ground sample was transferred into a ball mill jar, the ball-to-material ratio was 40:1, and the speed was set at 400 rpm for ball milling, and the ball milling was performed for 20 h to fully proceed the reaction. The reaction product was collected, washed with tetrahydrofuran, centrifuged, and repeated 4-5 times in order to ensure that all LiCl in the sa...
Embodiment 2
[0034] Preparation of Nano ZrB 2 .
[0035] The raw material used is: anhydrous ZrCl 4 (purity 98%), NaBH 4 (purity 98%), NaH (purity 95%), anhydrous NaCl (purity greater than 99%), glycerin (purity greater than 99%), ethanol (purity greater than 99%).
[0036] Molar ratio of ZrCl 4 : NaBH 4 : NaH = 1 : 2 : 2, weigh ZrCl in the glove box4 , NaBH 4 and NaH. Weigh anhydrous NaCl, its mass is 6 times of the total mass of the above three samples. ZrCl to be weighed 4 Grind evenly with anhydrous NaCl, then weigh the NaBH 4 and NaH into ZrCl 4 And NaCl mixture, further grinding evenly. The uniformly ground sample was transferred into a ball mill jar with a ball-to-material ratio of 30:1, and the ball milling speed was set at 380 rpm for ball milling, and the ball milling was carried out for 25 h to fully proceed the reaction. The reaction product is collected, washed alternately with glycerol and alcohol, centrifuged, and repeated 4-5 times in order to ensure that all N...
Embodiment 3
[0039] Preparation of nano-TiB 2 .
[0040] The raw material used is: TiCl 4 (purity 98%), LiBH 4 (purity 98%), LiH (purity 95%), anhydrous LiCl (purity greater than 99%), tetrahydrofuran (purity greater than 99%), metal Na (purity greater than 98%).
[0041] Molar ratio TiCl 4 : LiBH 4 : LiH = 1 : 2 : 3, weigh TiCl in the glove box 4 , LiBH 4 and LiH. Weigh anhydrous LiCl, whose mass is 8.5 times the total mass of the above three samples. LiBH to be weighed 4 Grind with LiH and anhydrous LiCl evenly, then transfer the above-mentioned uniformly ground sample into a ball mill jar, and add the weighed TiCl drop by drop 4 . The ball-to-material ratio was 40:1, and the set speed was 350 rpm for ball milling, and the ball milling was carried out for 30 h to fully proceed the reaction. The reaction product was collected, washed with tetrahydrofuran, centrifuged, and repeated 4-5 times in order to ensure that all LiCl in the sample was dissolved by tetrahydrofuran. Prio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com