Intermediary tetrasubstituted chlorin compound and application thereof in the field of medicines
A technology of chlorin and compounds, which is applied in the field of chlorin compounds, can solve the problems of insufficient stability and easy oxidation, and achieve the effects of improving stability, improving fat solubility and water solubility, and good selective absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 1. Synthesis experiment of raw materials
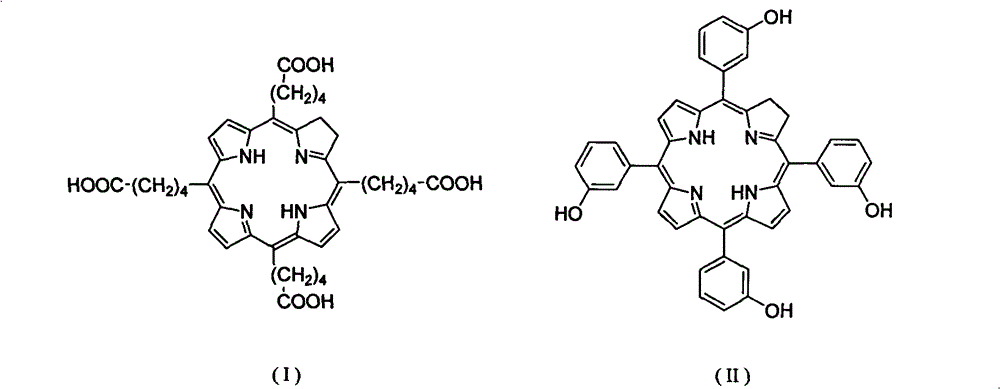
[0025] (1) 5,10,15,20-tetrakis-(4-methoxycarbonylbutyl)-porphine
[0026] Synthesis of (5,10,15,20-tetrakis(4-methoxycarbonylbutyl)-21H,23H-Porphine):
[0027]
[0028]
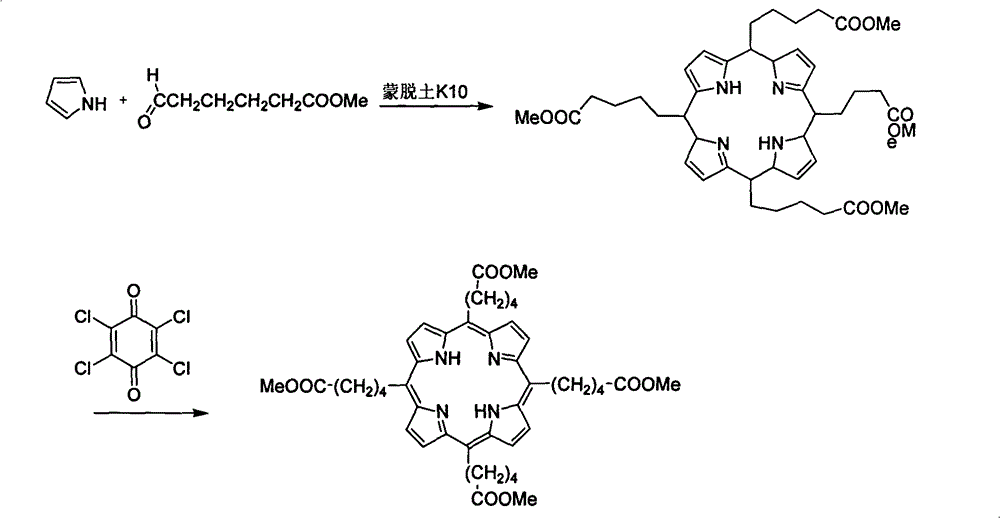
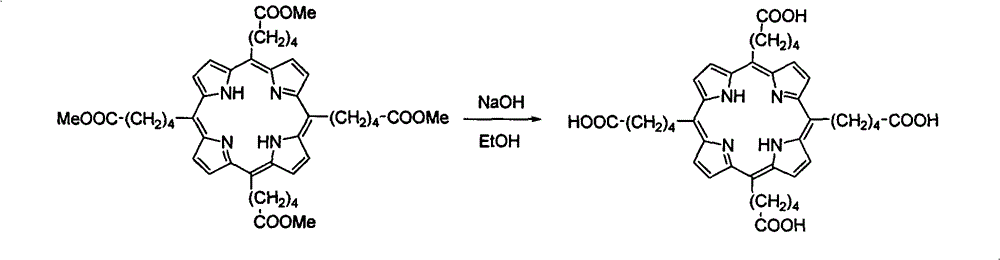
[0029] Weigh 12g of montmorillonite k10 into a 500mL three-neck flask and put it into an oven for activation at 120°C for 3 hours, then take it out, set up a reflux device, fill it with nitrogen, and wrap the bottle wall with aluminum foil to avoid light. Add 350 mL of dichloromethane and 10 mL of dichloromethane dissolved in 5-methoxycarbonyl-1-pentanal (3.2 g, 21 mmol), and stir for half an hour. With thorough stirring, pyrrole (1.52 mL, 22 mmol) was slowly added dropwise at room temperature, and stirring was continued for 1 hour. Then chloranil (3.87g, 15.75mmmol) was added and refluxed at 45°C for 1 hour. The solid powder was removed by filtration, and the filter cake was washed with ethyl acetate until it was colorless. The filtrate was spin-dri...
Embodiment 2
[0049] 1. Photodynamic anti-proliferation experiment of photosensitizers on colon cancer SW480 cells
[0050] Tested cells: colon cancer cells SW480
[0051] Test drug: a synthetic new photosensitizer, hematoporphyrin derivative HpD (produced by Beijing Institute of Pharmaceutical Industry).
[0052] Light source: MTZ-1 pulse laser cancer treatment machine; SD2490 laser power measuring instrument.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com