Preparation and refining method of repaglinide
A refining method and technology of repaglinide glutamate, which is applied in the field of preparation and refining of repaglinide, a drug for treating diabetes, can solve the problems of long reaction time, low yield, high cost, etc., and achieve simple operation and improved Purity and yield, reduced production costs and pollution effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] According to the preparation method of repaglinide of this embodiment, it comprises successively
[0033] Include the following steps:
[0034] Preparation of Regigamide (III) in Dichloromethane Solution
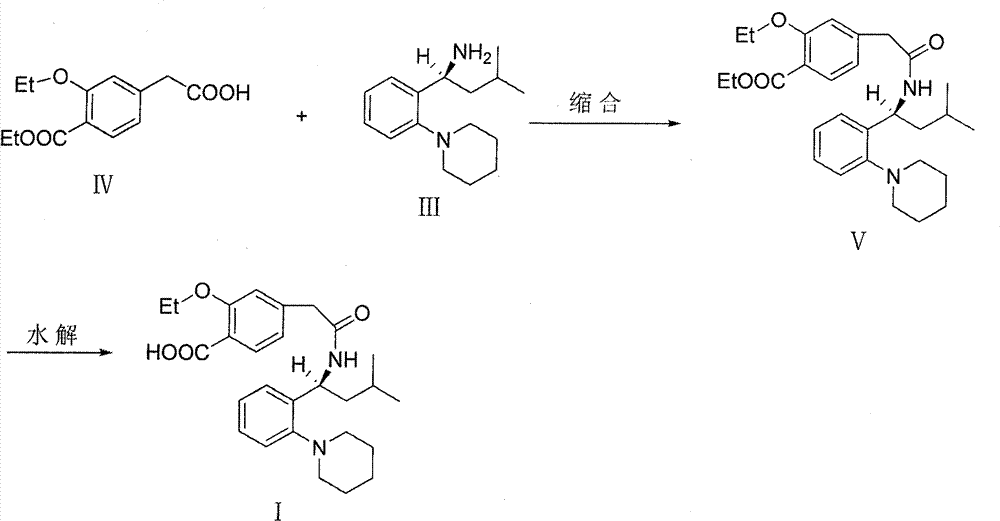
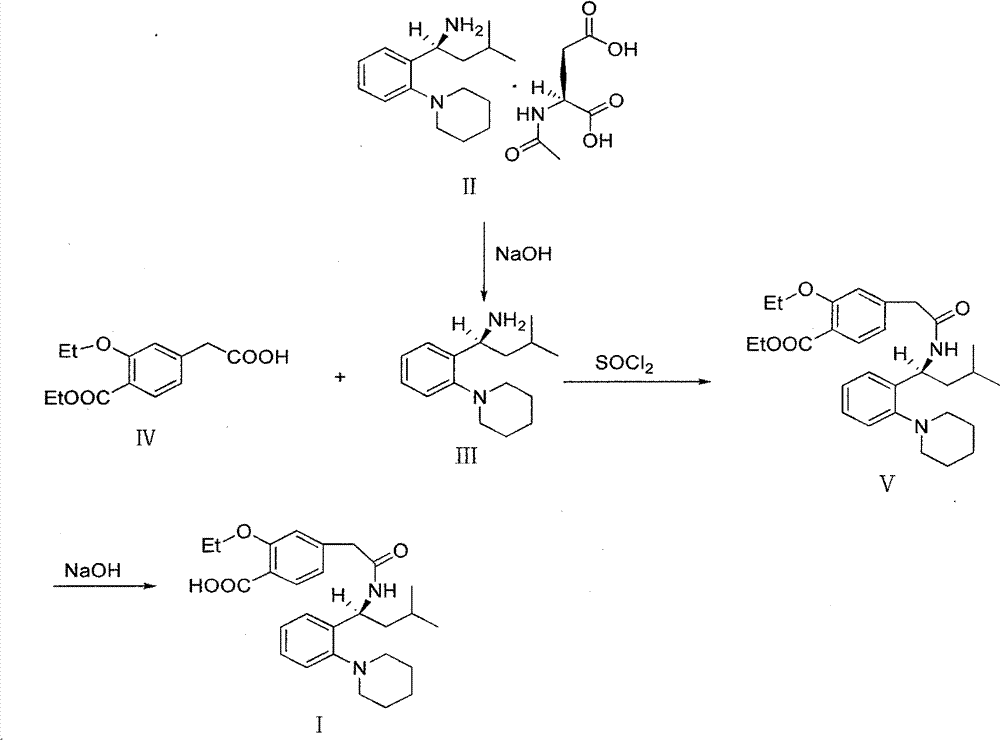
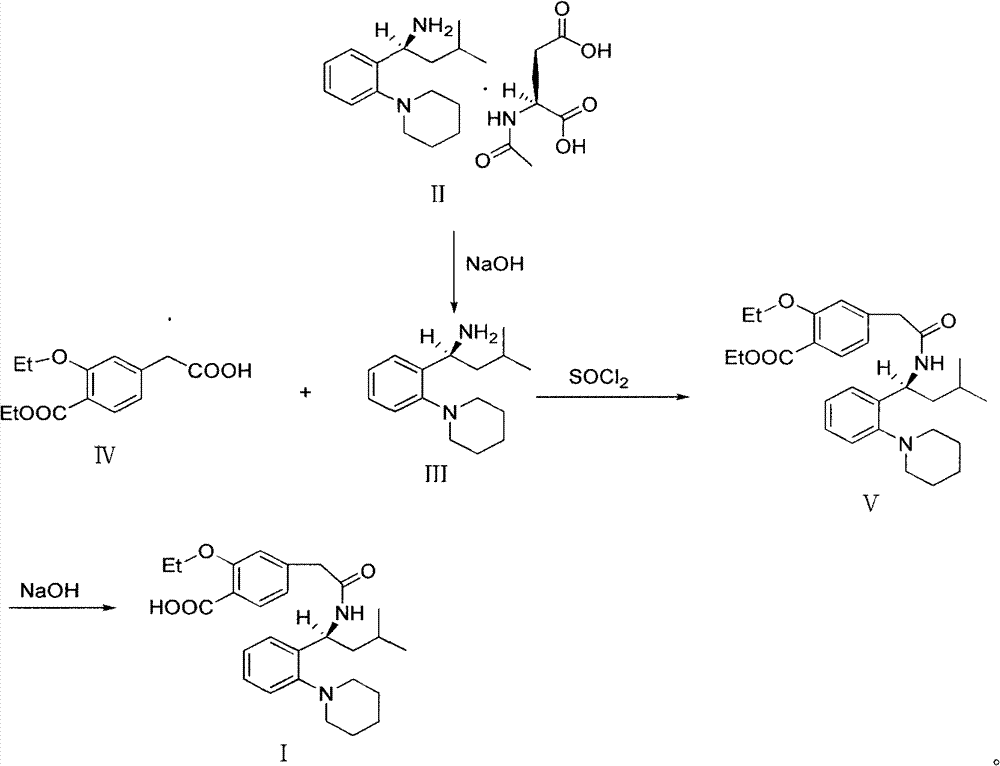
[0035] Stir to dissolve 19.6g of Regigamine glutamate and 80ml of dichloromethane, add dropwise the lye prepared by 6.37g of sodium hydroxide and 200ml of water; after the addition, stir at room temperature for 1-2 hours. Separate the layers, and extract the aqueous layer with 40ml of dichloromethane×2, combine the organic layers, and dry over anhydrous sodium sulfate; filter, remove about half of the solvent from the filtrate under reduced pressure at 30-35°C, and proceed directly to the next step. (TLC condition-chloroform:methanol=10:1);
[0036] Preparation of Ricolate (V)
[0037] Add 10g of Regalic acid (IV), 75ml of anhydrous dichloromethane, stir to dissolve, cool down to 2-6°C, add 0.1g of DMF, slowly add 9g of thionyl chloride dropwise, the dropwise addit...
Embodiment 2
[0044] According to the preparation method of repaglinide of the present embodiment, it comprises the following steps in sequence:
[0045] Preparation of Regigamide (III) in Dichloromethane Solution
[0046] 3.92kg of Regigamine glutamate and 16L of dichloromethane were stirred and dissolved, and the lye prepared by 1.28kg of sodium hydroxide and 40L of water was added dropwise; after the addition, stirred at room temperature for 1 to 2 hours. The layers were separated, and the aqueous layer was decompressed with 8 L x 2 dichloromethane filtrate at 30-35°C to remove about half of the solvent, and directly proceeded to the next step. (TLC conditions - chloroform: methanol = 10:1).
[0047] Preparation of Ricolate (V)
[0048] Add 2kg of Regalic acid (IV), 15L of anhydrous dichloromethane, stir to dissolve, cool down to 2-6°C, add 20g of DMF, slowly add 1.8kg of thionyl chloride dropwise, about 0.5-1h to complete the dropwise addition, and the dropwise addition is complete ,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com