Halogen-free resin composition, bonding sheet and copper-clad laminate
A resin composition and bonding sheet technology, applied in the field of copper clad laminates, can solve the problems of poor mechanical properties, reduced halogen content, high water absorption, etc., and achieve high glass transition temperature, excellent heat resistance, good The effect of flame retardant effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The preparation method of described benzoxazine resin is, adopts solution synthesis method, and it comprises steps as follows:
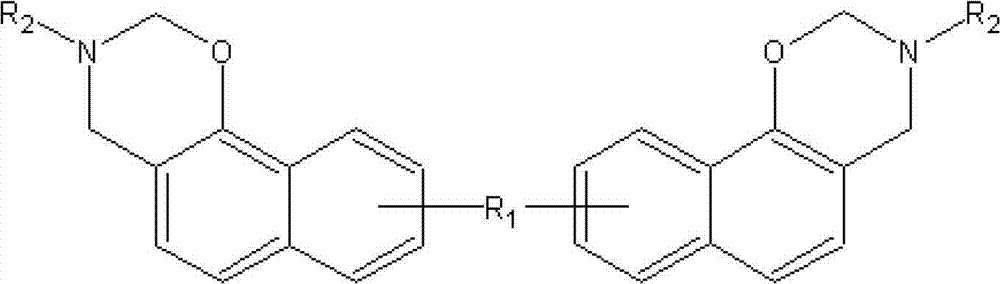
[0031] Step 1, phenol, primary amine, aldehyde are quantified as 1: (0.8-1.2): (1.5-2.5) by phenolic hydroxyl group, amine group, aldehyde functional group molar ratio; The structural formula of described phenol is as follows:
[0032] (Formula 2)
[0033] In formula 2, R1 is O, C=O, S, SO2, alicyclic hydrocarbons with 3 to 20 carbon atoms and derivatives thereof, aliphatic hydrocarbons with 1 to 20 carbon atoms and derivatives thereof, or alicyclic hydrocarbons with 2 to 20 carbon atoms 20 unsaturated aliphatic hydrocarbons and their derivatives. The primary amine is: NH2R2, wherein R2 is an alicyclic hydrocarbon with 3 to 20 carbon atoms and its derivatives, an aliphatic hydrocarbon with 1 to 20 carbon atoms and its derivatives, or an unsaturated fat with 2 to 20 carbon atoms Hydrocarbons and their derivatives, such as allylamine, anilin...
preparation example 1
[0044] Preparation of Benzoxazine Resin by Solution Synthesis
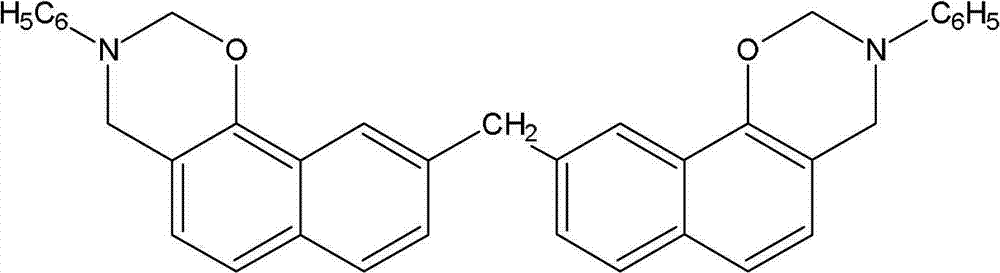
[0045]Dissolve phenol in absolute ethanol at room temperature, adjust the pH to 8-10, and add toluene, aniline and formaldehyde aqueous solution at a molar ratio of phenolic hydroxyl, amino and aldehyde functional groups of 1:1:2. After stirring evenly, heat up to reflux, react for 4 hours and distill ethanol and water toluene under reduced pressure, cool the device, add butanone to obtain a light yellow translucent viscous body, after washing, purification and drying, the benzoxazine resin is obtained , and the yield was 80.5%. The structural formula of the obtained benzoxazine resin is as follows:
[0046]
preparation example 2
[0048] Preparation of Benzoxazine Resin by Solution Synthesis
[0049] Dissolve phenol in absolute ethanol at room temperature, adjust the pH to 8-10, and add toluene, aniline and paraformaldehyde at a molar ratio of phenolic hydroxyl, amino, and aldehyde functional groups of 1:1.2:2.5. After stirring evenly, heat up to reflux, react for 4 hours, and distill ethanol, water, and toluene under reduced pressure, and cool the device to obtain a light yellow translucent viscous body. After washing, purification, and drying, the benzoxazine resin is obtained. The rate is 78.8%. The structural formula of the obtained benzoxazine resin is as follows:
[0050]
PUM
| Property | Measurement | Unit |
|---|---|---|
| epoxy equivalent | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com