Red phosphor and preparation method thereof
A red phosphor and compound technology, applied in the field of red phosphor and its preparation, can solve the problems of high production cost, low color rendering index, and great environmental hazards of matrix materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Select strontium carbonate, aluminum oxide, manganese carbonate and boric acid as the starting compound raw materials, and weigh four kinds of compound raw materials respectively according to the molar ratio of each element, totally 8 groups, and the ratio is as follows:
[0035] (1) Sr:Al:Mn:B=4:13.986:0.014:0.07, corresponding to x=0.1%, y=0.5%;
[0036] (2) Sr:Al:Mn:B=4:13.986:0.014:0.70, corresponding to x=0.1%, y=5%;
[0037] (3) Sr:Al:Mn:B=4:13.986:0.014:1.40, corresponding to x=0.1%, y=10%;
[0038](4) Sr:Al:Mn:B=4:13.986:0.014:2.10, corresponding to x=0.1%, y=15%;
[0039] (5) Sr:Al:Mn:B=4:13.986:0.014:2.80, corresponding to x=0.1%, y=20%;
[0040] (6) Sr:Al:Mn:B=4:13.986:0.014:3.50, corresponding to x=0.1%, y=25%;
[0041] (7) Sr:Al:Mn:B=4:13.986:0.014:4.20, corresponding to x=0.1%, y=30%;
[0042] (8) Sr:Al:Mn:B=4:13.986:0.014:5.60, corresponding to x=0.1%, y=40%;
[0043] The total weight of the control mixture is 20 grams. After grinding and mixing 20 ...
Embodiment 2
[0050] Select strontium bicarbonate, aluminum nitrate, manganous oxide and diboron trioxide as starting materials, and weigh four kinds of compound raw materials respectively according to the molar ratio of each element, altogether 3 groups, and the ratio is as follows:
[0051] (1) Sr:Al:Mn:B=4:13.9986:0.0014:0.70, corresponding to x=0.01%, y=5.0%;
[0052] (2) Sr:Al:Mn:B=4:13.9860:0.014:0.70, corresponding to x=0.10%, y=5.0%;
[0053] (3) Sr:Al:Mn:B=4:13.3000:0.70:0.70, corresponding to x=5.0%, y=5.0%;
[0054] The total weight of the control mixture is 20 grams. After grinding and mixing 20 grams of the mixture, put it into a corundum crucible, and then put the crucible into a high-temperature electric furnace. Precisely controlling the heating rate, the sample was pre-fired at 500°C for 10 hours. Take out the pre-fired sample, grind and mix it again, put it into the crucible, and burn it at 1300°C for 5 hours under the air atmosphere, after grinding it again, burn it at...
Embodiment 3
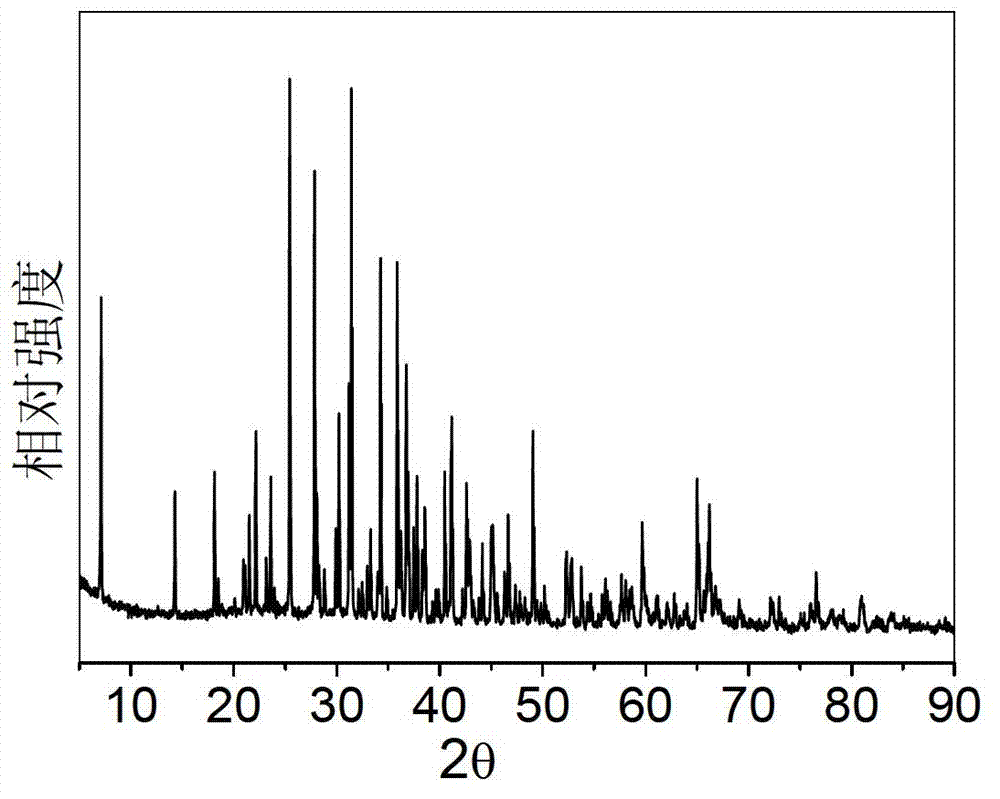
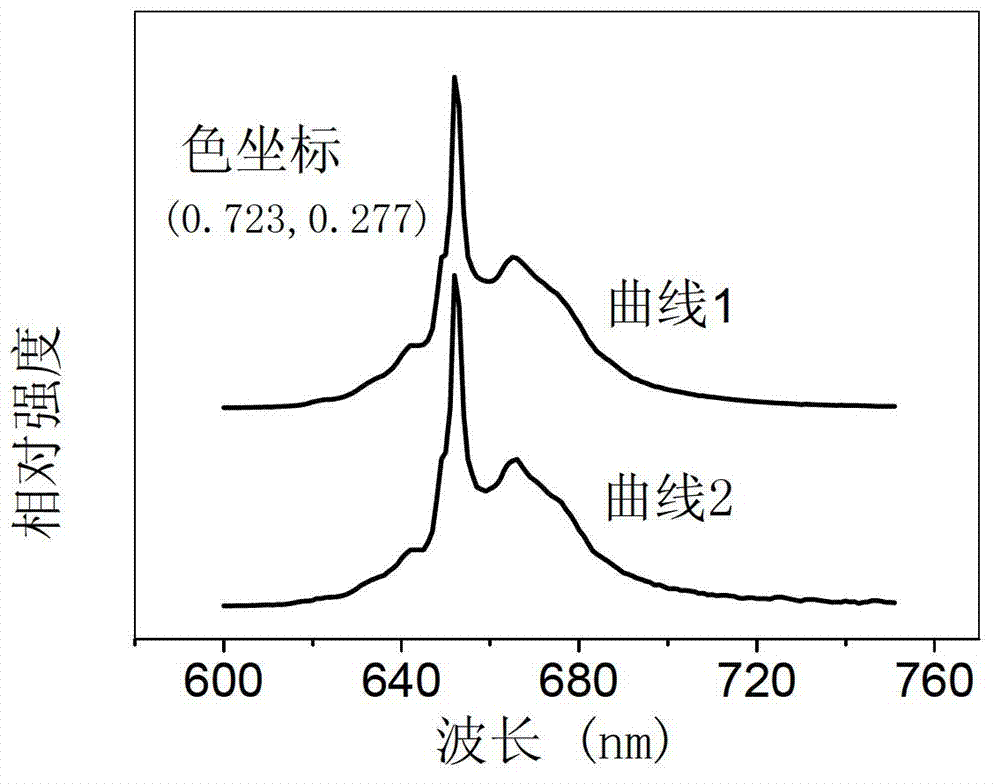
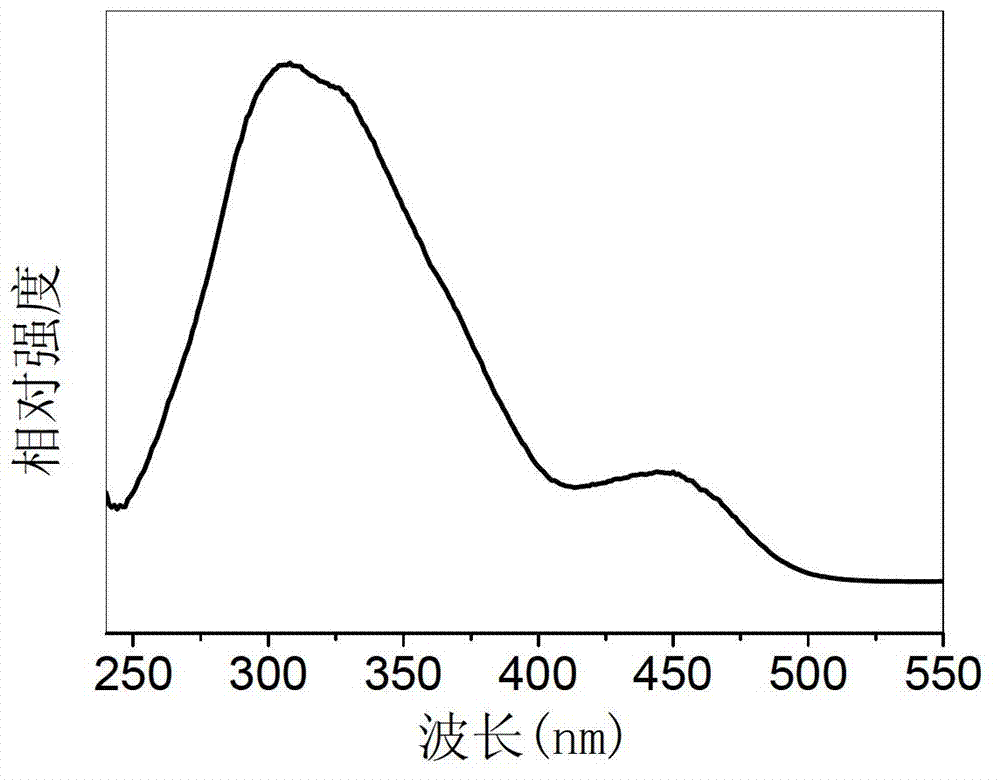
[0057] Select strontium oxide, aluminum hydroxide, manganese oxide and boric acid as starting materials, according to the molar ratio of each element Sr:Al:Mn:B=4:13.9860:0.014:0.70, corresponding to x=0.10%, y=5.0%; Four kinds of raw materials were weighed respectively, and the total weight of the control mixture was 20 grams. After grinding and mixing 20 grams of the mixture, put it into a corundum crucible, and then put the crucible into a high-temperature electric furnace. Precisely controlling the heating rate, the sample was pre-fired at 1000°C for 5 hours. The pre-fired sample is taken out, ground and mixed again, burned at 1600°C for 2 hours in an air atmosphere, taken out and ground again, burned at 1600°C for 2 hours in an air atmosphere, and cooled naturally with the furnace, that is The tetravalent manganese ion-doped strontium aluminate red fluorescent material is prepared. X-ray diffraction analysis showed that it was Sr 4 al 14 o 25 :Mn 4+ crystal phase. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com