Methods for producing nerve cells from stem cells, nerve cells and uses thereof
A nerve cell and stem cell technology, applied in the field of cell transplantation, can solve the problem that nerve cells are not suitable for clinical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Example 1 : Isolation of human placental mesenchymal stem cells
[0079] The placenta used in this experiment was the term placenta of healthy puerpera, which was obtained from the Obstetrics Department of the Affiliated Hospital of Ningxia Medical University. The donors voluntarily donated without compensation and signed the informed consent. Approximately 20 g of fresh human placental tissue was dissected from the postpartum placenta under sterile conditions. The tissue was preserved in a 50 ml centrifuge tube containing 20 ml of DMEM (Invitrogen, product number 11885084, product number 11885084 ) culture medium. Placental tissue in the above protection solution was transferred to a cGMP laboratory within 30 minutes. Prior to processing, the tissue was washed three times in PBS (Invitrogen, product number 14040133) containing 1% penicillin / streptomycin.
[0080] Dissect the chorionic disc from the placental tissue using sterile surgical scissors, wash the chorion...
Embodiment 2
[0081] Example 2: Embryonic brain proteins induce mesenchymal stem cells to differentiate into neural cells
[0082] The steps of inducing mesenchymal stem cells to differentiate into nerve cells include:
[0083] 1. The human placental mesenchymal stem cells (MSCs) obtained in Example 1 were cultured in a standard medium (DMEM+10% FBS) to a density of 80%.
[0084] 2. The cells were digested with 200u / ml collagenase IV (Invitrogen) at 37°C for 3-5 minutes to obtain single cells.
[0085] 3. Transfer the cells into collagen-IV (Invitrogen)-coated culture flasks, add standard DMEM medium and culture for 24 hours.
[0086] 4. Replace the medium with fresh medium (DMEM+20% FBS) and culture for 16 hours.
[0087] 5. Continue culturing the cells in the initial differentiation medium for 16 hours, wherein the initial differentiation medium is a standard DMEM / F12 medium containing one-fold concentration of N2 mixed cofactor (Invitrogen), 2ug / ml heparin ( Heparin, Sigma), 1% Mini...
Embodiment 3
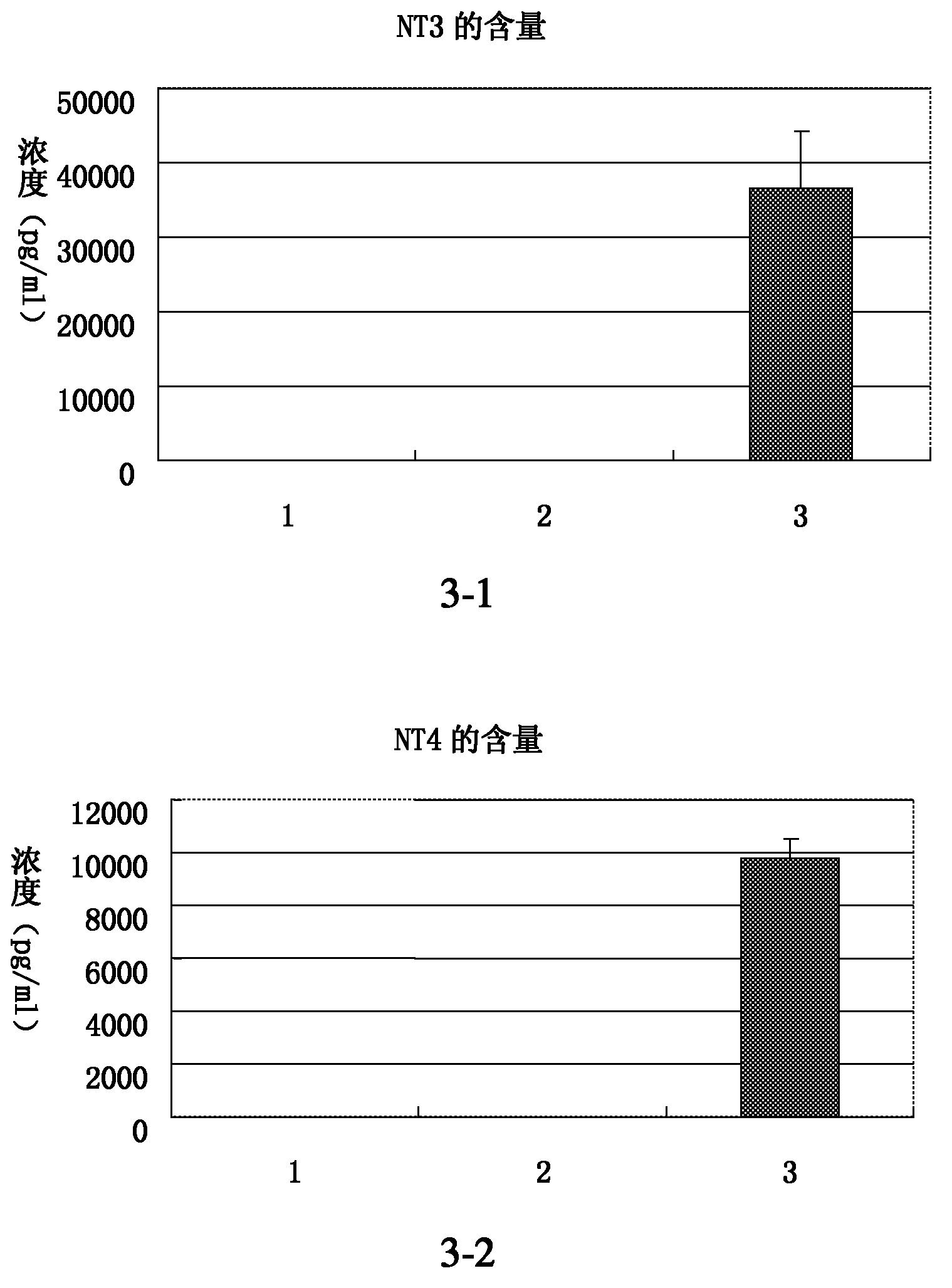
[0092] Example 3: Determination of neuron-specific proteins
[0093] The assay steps include:
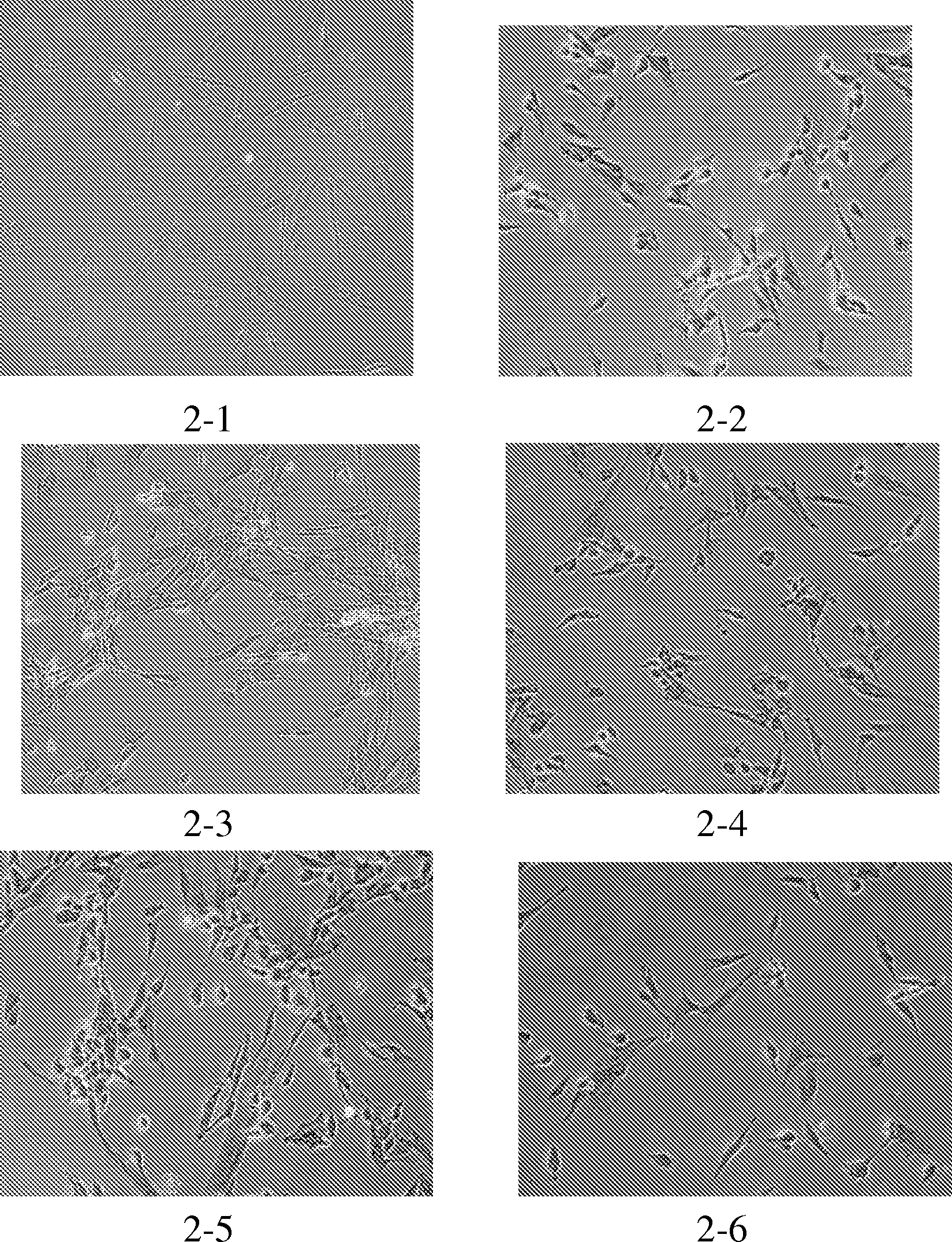
[0094] 1, cell climbing sheet: the nerve cell (test group) that obtains from embodiment 3 is divided into 3 * 10 5 Densities were transferred to culture dishes with coverslips and cultured in neuronal culture medium (same as in Example 3, Step 6). In addition, uninduced human placental mesenchymal stem cells were cultured under the same conditions as a control group. The test group and the control group received exactly the same treatment in all the following steps.
[0095] 2. After 24 hours, wash the cells on the glass slide with cells (neuroid cells and control cells respectively) with PBS for 3 times, 5 minutes each time.
[0096] 3. Add 30% H to the slide 2 o 2 (1 part) and pure methanol (50 parts) for 30 minutes, and then washed twice with distilled water.
[0097] 4. Add a blocking solution containing 5% bovine serum albumin (BSA) dropwise on the cells of the glass sl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com