Palladium catalyst for catalyzing Suzuki coupling reaction, synthesis method, application and ligand
A technology of palladium catalyst and synthesis method, applied in the direction of physical/chemical process catalyst, organic compound/hydride/coordination complex catalyst, chemical instrument and method, etc., can solve the problems of oxidation and toluene toxicity, and reach the reaction temperature and time reduction, the effect of less alkali usage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The synthesis route of the catalyst synthesized by the present invention is as follows:
[0049] 1. Preparation of Ligand L:
[0050]
[0051]Weigh 2.94g (10mmol) of intermediate A into a 100ml round bottom flask, add 20ml of thionyl chloride, and reflux until the system is clarified. First evaporate most of the thionyl chloride at normal pressure, then add two droppers of benzene, evaporate to dryness under reduced pressure, repeat three times, finally add two droppers of anhydrous ether, and evaporate to dryness under reduced pressure to obtain a yellow solid in a flask for later use. Intermediate B. Intermediate A References Sather, A.C., Berryman, O.B., Rebek, J.Jr.J.AM. Chem Soc, 2010, 132, 13572-13574; Kolotuchin, V.S., Thiessen, A.P., Fenlon, E.T., Wilson, R.S., Loweth, J.C., Zimmerman, C.S. Chem. Eur. J. 1999, 5, 2537-2547.
[0052]
[0053] Add 4.41g of 5-(4-pyridyl)tetrazole and 10ml of anhydrous pyridine to the prepared intermediate B, and react at 1...
experiment example 1
[0056] Experimental Example 1: PhBr and PhB(OH) 2 Reaction to form biphenyl
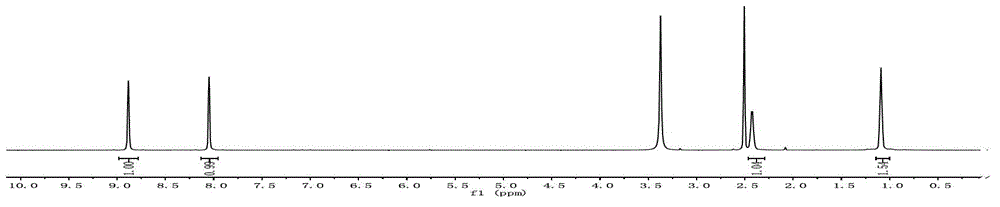
[0057] Add Ph-Br (10mmol), PhB (OH) to a 25ml single-necked round bottom flask 2 (12mmol), K 2 CO 3 (10mmol), catalyst 1mg (100ppm), H 2 O / EtOH 5ml (1 / 1), oil bath temperature control 60 ℃, constant temperature reaction 1h. Stop heating, add 5ml H to the system 2 O, extracted with 3 x 10 ml ethyl acetate, combined organic phases, MgSO 4 Dry, spin dry, and use Dichloromethane / Petroleun ether=1:1 column chromatography to obtain a white product with a yield of 95%. The H NMR of the product is as Figure 7 As shown, IR as Figure 8 shown.
[0058] Traditional Pd (PPh 3 ) 4 The amount of catalyst used in catalyzing this type of reaction is usually 1% to 5%. See references Castanet, A.S.; Colobert, F.; Schlama, T.Org.Lett.2000, 23, 3559-3561. 1 mg of new catalyst, the amount of Pd is 0.1 mg. while Pd (PPh 3 ) 4 The catalyst requires 115.6 mg based on 1%, and the amount of Pd is 10.6 mg.
experiment example 2
[0059] Experimental Example 2: PhI and PhB(OH) 2 Reaction to form biphenyl
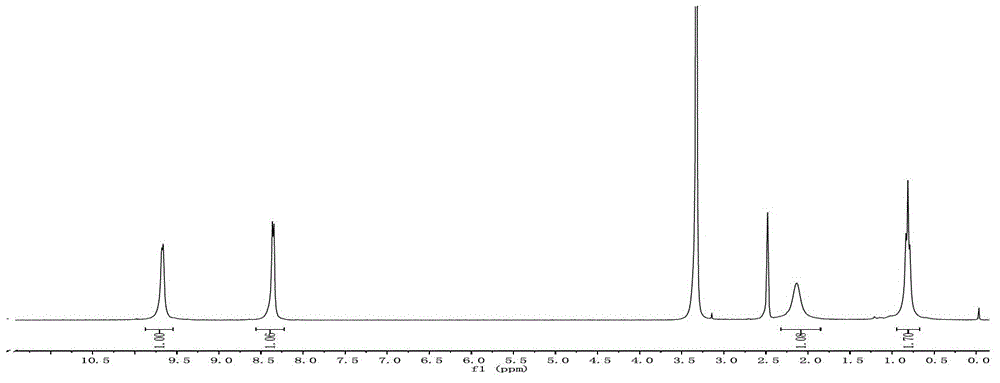
[0060] Add Ph-I (10mmol), PhB (OH) to a 25ml single-necked round bottom flask 2 (12mmol), K 2 CO 3 (10mmol), catalyst 1mg (100ppm), H 2 O / EtOH 5ml (1 / 1), oil bath temperature control 60 ℃, constant temperature reaction 1h. Stop heating, add 5ml H to the system 2 O, extracted with 3 x 10 ml ethyl acetate, combined organic phases, MgSO 4 Dry, spin dry, and use Dichloromethane / Petroleun ether=1:1 column chromatography to obtain a white product with a yield of 96%. H NMR of the product as Figure 9 As shown, IR as Figure 10 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com