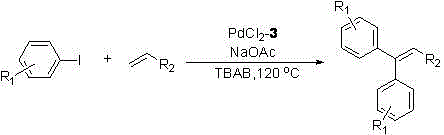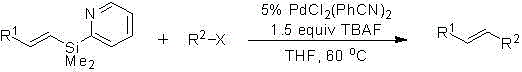Method for preparing substituted alkene
A technology for olefins and halogenated hydrocarbons is applied in the field of catalytic coupling synthesis of substituted olefins to achieve the effects of easy availability of raw materials, simple operation and convenient post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Pd(dppf) was sequentially filled in a Schlenk test tube 2 Cl 2 (0.015 mmol), triethylamine (1.0 mmol), potassium vinylfluoroborate (0.5 mmol) and DMF (2 mL), and iodobenzene (0.5 mmol) was added with a microinjector. Then the system was sealed and heated in an oil bath at 120°C for about 12 hours, then p-methoxyiodobenzene (0.5 mmol) was added, and the system was sealed and heated in an oil bath at 120°C for about 24 hours. The system was then allowed to cool to room temperature, 2 mL of water was added to quench the reaction, and then extracted with ethyl acetate (4 mL×3), the organic phases were combined and dried over anhydrous sodium sulfate, concentrated and passed through simple column chromatography (eluent used Petroleum ether:ethyl acetate=24:1) to obtain the coupling product 1-phenyl 2-p-methoxyphenylethylene (85.2 mg), with a yield of 81%. Its NMR data are: 1 H NMR (400 MHz, CDCl 3 ) (δ, ppm) 3.81 (s, 3H), 6.89 (d, J = 8.4 Hz, 2H), 6.96 (d, J = 16.0 Hz, ...
Embodiment 2
[0034] Pd(dppf) was sequentially filled in a Schlenk test tube 2 Cl 2 (0.015 mmol), triethylamine (1.0 mmol), potassium vinylfluoroborate (0.5 mmol) and DMF (2 mL), and p-chloroiodobenzene (0.5 mmol) was added with a microinjector. Then the system was sealed and heated in an oil bath at 120°C for about 12 hours, then p-fluoroiodobenzene (0.5 mmol) was added, and then the system was sealed and heated in an oil bath at 120°C for about 24 hours. The system was then allowed to cool to room temperature, and 2 mL of water was added to quench the reaction, followed by extraction with ethyl acetate (4 mL × 3), the combined organic phases were dried over anhydrous sodium sulfate, concentrated and passed through simple column chromatography (eluent used Petroleum ether:ethyl acetate=24:1) to obtain the coupling product 1-p-chlorophenyl 2-p-fluorophenylethylene (97.4 mg), with a yield of 84%. Its NMR data are: 1 H NMR (400 MHz CDCl 3) 6.98 (d, J = 16.4, 1H), 7.04-7.09 (m, 3H), 7.35 ...
Embodiment 3
[0036] Pd(dppf) was sequentially filled in a Schlenk test tube 2 Cl 2 (0.015 mmol), triethylamine (1.0 mmol), potassium vinylfluoroborate (0.5 mmol) and DMF (2 mL), and p-fluoroiodobenzene (0.5 mmol) was added with a microinjector. Then the system was sealed and heated in an oil bath at 120°C for about 12 hours, then p-methoxyiodobenzene (0.5 mmol) was added, and the system was sealed and heated in an oil bath at 120°C for about 24 hours. The system was then allowed to cool to room temperature, 2 mL of water was added to quench the reaction, and then extracted with ethyl acetate (4 mL×3), the organic phases were combined and dried over anhydrous sodium sulfate, concentrated and passed through simple column chromatography (eluent used Petroleum ether:ethyl acetate=24:1) to obtain the coupling product 1-p-methoxyphenyl 2-p-fluorophenylethylene (92.4 mg), with a yield of 81%. Its NMR data are: 1 H NMR (400 MHz CDCl 3 ) 3.84 (s, 3H), 6.84-6.96 (m, 4H), 7.03 (t, J = 8.8, 2H), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com