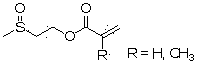
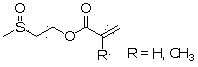
Acrylate compound containing methyl sulfoxide structure, and preparation method and application thereof
A technology of acrylate and methyl sulfoxide, applied in the field of acrylate compound containing methyl sulfoxide structure and its preparation, can solve the problems of low volatility, affect the performance of cured film, difficult to disperse, etc., and achieve good solubility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
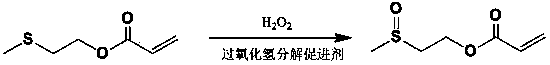
[0034] Take 81 g of 2-methylthioethyl acrylate and place it in a 500 ml round bottom flask, add 80 ml of hydrogen peroxide with a mass concentration of 30%, 2.1 g of copper chloride, and 2 g of cetyltrimethylammonium bromide , stirred at room temperature for 3 hours, added 14 g of sodium dithionite, added an appropriate amount of saline to separate layers, removed water, washed the product twice with water, dried over anhydrous sodium sulfate, and obtained the formula (I) of R=H Product 87.3 g. 1 H NMR (CDCl 3 , ppm): 2.47 (3H, s), 2.88 (2H, t), 4.40 (2H, t), 5.78 (1H, m), 6.11 (1H, m), 6.33 (1H, m). ESI-MS m / z: 162.04.
[0035] Calculated by MOPAC software, the GAMESS Interface theoretical dipole moment of the compound of formula (I) prepared in Example 1 is 6.12 Debye.
Embodiment 2
[0037] Take 81 g of 2-methylthioethyl acrylate and place it in a 500 ml round-bottomed flask, add 80 ml of mass concentration of 30% hydrogen peroxide, 2.1 g of copper sulfate, and 2 g of cetyltrimethylammonium bromide, Stir the reaction at room temperature for 3 hours, add 14 g of sodium dithionite, add an appropriate amount of saline to separate layers, remove water, wash the product twice with water, and dry it with anhydrous sodium sulfate to obtain the formula (I) 87 of R=H g.
Embodiment 3
[0039] Take 81 g of 2-methylthioethyl acrylate and place it in a 500 ml round bottom flask, add 80 ml of 30% hydrogen peroxide, 2.3 g of copper acetate, and 2 g of cetyltrimethylammonium bromide, Stir the reaction at room temperature for 3 hours, add 14 g of sodium dithionate, add an appropriate amount of saline to separate layers, remove water, wash the product twice with water, and dry it with anhydrous sodium sulfate to obtain the formula (I) 88.2 of R=H g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com