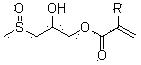
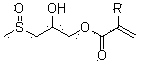
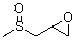Compound containing methyl sulfoxide structure, and preparation method and application thereof
A technology for methyl sulfoxide and compounds is applied in the field of compounds containing methyl sulfoxide structures and their preparation, which can solve the problems of low volatility, residual toxicity of solvents, osmotic migration and the like, and achieve the effect of good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Take 88 g of allyl methyl sulfide and place it in a 1000 ml round bottom flask, add 555 ml of 30% hydrogen peroxide, 6.2 g of formic acid peroxide, and 39 g of cetyltrimethylammonium bromide, and stir at room temperature After reacting for 4 hours, add 180 g of sodium hydrosulfate, add an appropriate amount of saline to separate layers, remove water, wash the product twice with water, and dry over anhydrous sodium sulfate to obtain 102.6 g of the compound of formula (II), 1 H NMR (CDCl 3 , ppm): 2.61 (3H, s), 2.52 ~ 2.79 (2H, d-d), 2.89 (2H, m), 2.93 (1H, m) ESI-MS m / z: 120.14.
[0040] Take 60 g of the compound of formula (II) and place it in a 250 ml three-necked flask, add 37 g of acrylic acid, 0.5 g of tetrabutylammonium bromide, and 0.6 g of p-methoxyphenol, raise the temperature to 75°C, stir and heat the reaction, and measure When the acid value drops below 6 mgKOH / g, the reaction is stopped to obtain the product methyl sulfoxide acrylate (I). 1 H NMR (CDCl 3 ...
Embodiment 2
[0043]Take 88 g of allyl methyl sulfide and place it in a 1000 ml round bottom flask, add 295 ml of 30% hydrogen peroxide, 34.5 g of acetic acid peroxide, and 17 g of cetyltrimethylammonium bromide, and stir at room temperature After reacting for 5 hours, add 180 g of sodium dithionite, add salt until the layers are separated, remove water, wash the product twice with water, and dry over anhydrous sodium sulfate to obtain 93.2 g of the compound of formula (II).
[0044] Take 60 g of the compound of formula (II) and place it in a 250 ml three-necked flask, add 37 g of acrylic acid, 0.5 g of tetrabutylammonium chloride, and 0.6 g of p-methoxyphenol, raise the temperature to 75°C, stir and heat the reaction, and measure When the acid value drops below 6 mgKOH / g, the reaction is stopped to obtain the product methyl sulfoxide acrylate (I).
Embodiment 3
[0046] Take 88 g of allyl methyl sulfide and place it in a 1000 ml round bottom flask, add 295 ml of 30% hydrogen peroxide, 25 g of formic acid peroxide, and 5.5 g of cetyltrimethylammonium bromide, and stir at room temperature After reacting for 5 hours, add 180 g of sodium dithionite, add salt until the layers are separated, remove water, wash the product twice with water, and dry over anhydrous sodium sulfate to obtain 112.0 g of the compound of formula (II).
[0047] Take 60 g of the compound of formula (II) and place it in a 250 ml three-necked flask, add 37 g of acrylic acid, 0.5 g of tetramethylammonium bromide, and 0.6 g of p-methoxyphenol, raise the temperature to 75°C, stir and heat the reaction, and measure When the acid value drops below 6 mgKOH / g, the reaction is stopped to obtain the product methyl sulfoxide acrylate (I).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com