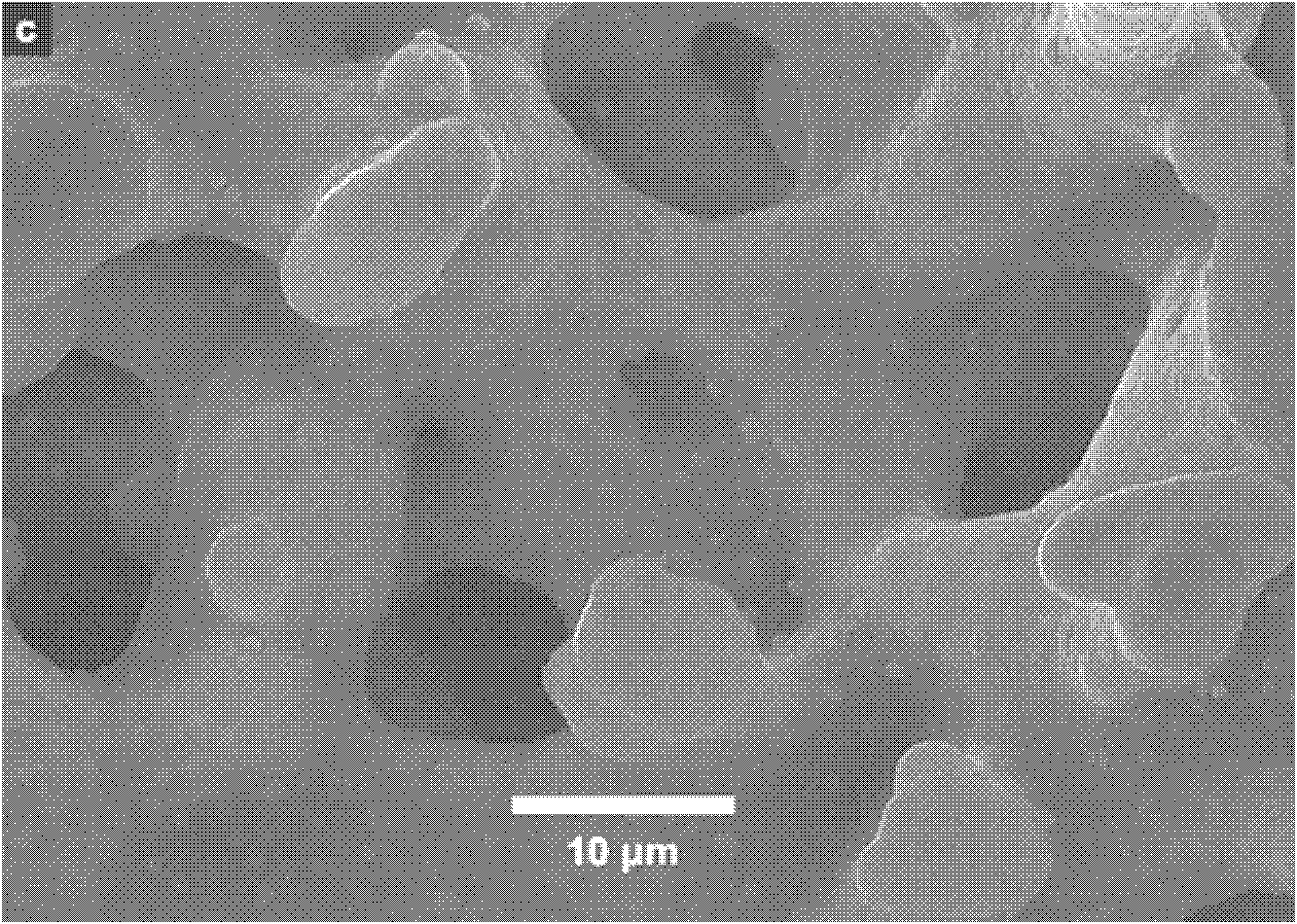Diplopore-structured arsenic adsorption material and preparation method thereof
A technology of adsorption material and pore structure, applied in chemical instruments and methods, adsorption water/sewage treatment, inorganic chemistry, etc., can solve problems such as unsuitability for various water processors, small arsenic exchange capacity, interference of arsenic adsorption, etc., to achieve Avoid the loss of effective ingredients, fast adsorption, and reduce the effect of mass transfer resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of silica support
[0037] First, tetraethyl orthosilicate, water, nitric acid, and polyethylene glycol are mixed and stirred at a mass ratio of 12.26:17.2:1.38:1, hydrolyzed at room temperature, and then placed at 40 degrees Celsius for gel-phase The reaction was separated to obtain a silica framework with a macropore diameter of 10 μm, moderate strength, and able to withstand a certain pressure, in which the mass fraction of nitric acid was 36%.
[0038] Preparation of mesoporous
[0039]Immerse the obtained silica skeleton with a macropore diameter of 10 μm in 0.5 mol / L ammonia water and treat it at 120 degrees Celsius for 9 hours, take it out, dry it, and calcinate to obtain a silica carrier with a double-pore structure, with a macropore diameter of 10 μm and a mesopore diameter of 10 μm. 23nm, the specific surface area is 193m 2 / g.
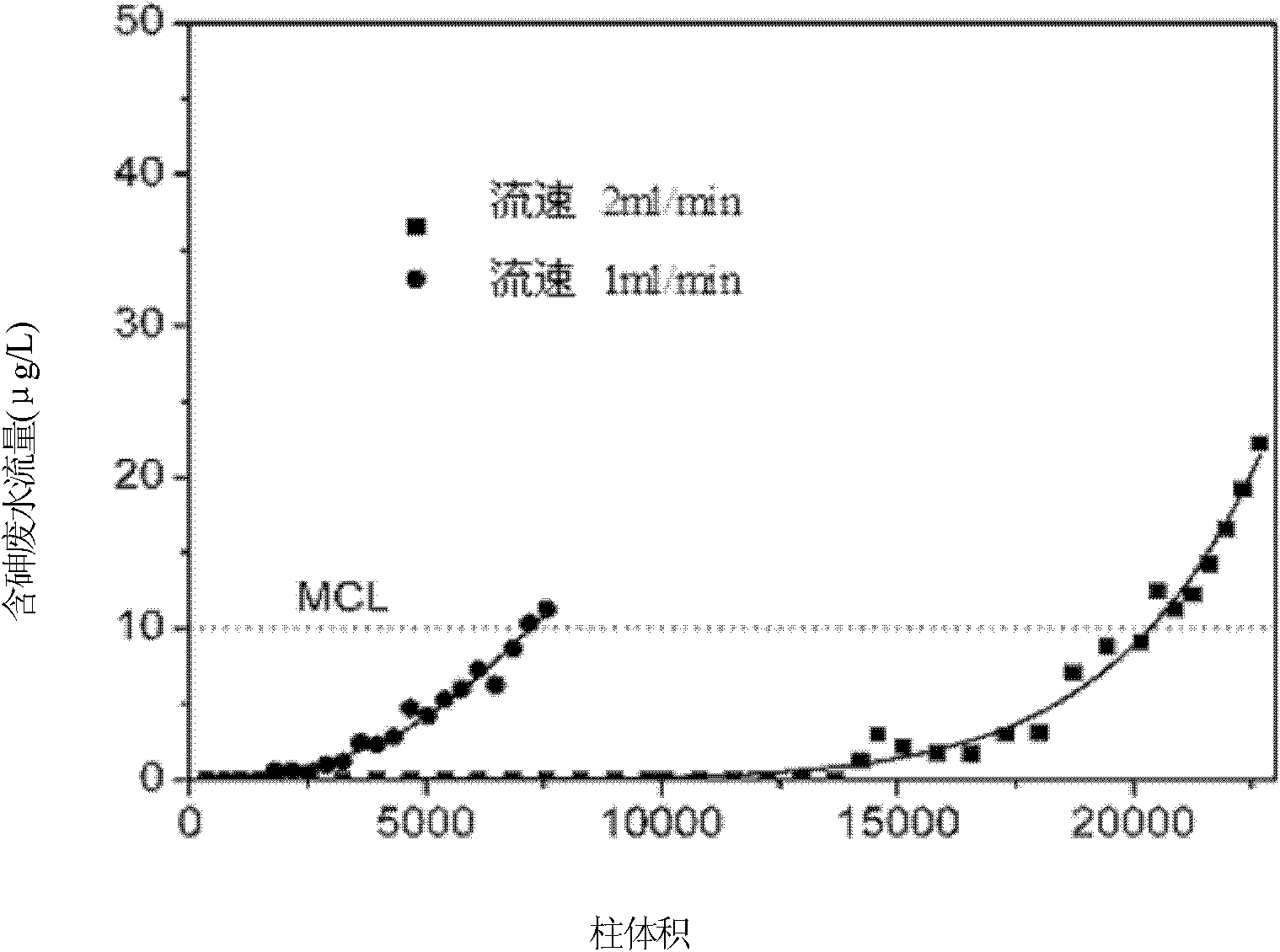
[0040] The obtained macropore diameter is 10 μm, and the silica carrier with a dual-pore structure with a mesopore di...
Embodiment 2
[0043] Preparation of silica support
[0044] The difference from Example 1 is that tetraethyl orthosilicate, water, nitric acid, and polyethylene glycol are mixed and stirred at a ratio of 12.26:17.2:1.38:1, hydrolyzed at room temperature, and placed under the condition of 41 degrees Celsius after hydrolysis is complete. The gel-phase separation reaction is carried out to obtain a silica framework with a macropore diameter of 7 μm, which has moderate strength and can withstand a certain pressure.
[0045] Preparation of mesoporous
[0046] The obtained silica skeleton with a macropore diameter of 7 μm was immersed in 0.2 mol / L ammonia water at 120 degrees Celsius for 9 hours, and then dried and calcined to obtain a dual-porous silica carrier with a macropore diameter of 7 μm and a mesopore diameter of 13nm, the specific surface area is 412m 2 / g.
[0047] Immerse the silica carrier with a dual-pore structure in a cerium nitrate solution with a concentration of 500g / L, soak...
Embodiment 3
[0049] Preparation of silica support
[0050] The difference from Example 1 is that tetraethyl orthosilicate, water, nitric acid, and polyethylene glycol are mixed and stirred at a ratio of 12.26:17.2:1.38:1, hydrolyzed at room temperature, and placed under the condition of 42 degrees Celsius after hydrolysis is complete. The gel-phase separation reaction is carried out to obtain a silica framework with a macropore diameter of 1.8 μm, which has moderate strength and can withstand a certain pressure.
[0051] Preparation of mesoporous
[0052] The obtained silica skeleton with a macropore diameter of 1.8 μm was immersed in 0.2mol ammonia water at 80°C for 9 hours, then dried and calcined to obtain a silica carrier with a dual-pore structure, with a macropore diameter of 1.8 μm and a mesopore diameter of 15nm, the specific surface area is 320m 2 / g.
[0053] Immerse the silica carrier with a dual-pore structure in a cerium nitrate solution with a concentration of 2000g / L, soa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com