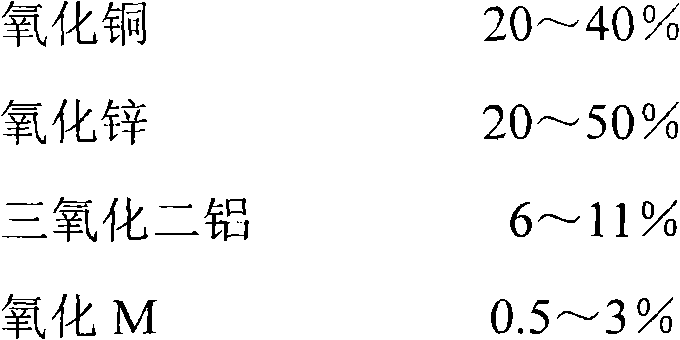Preparation method of environmentally-friendly low-temperature shift catalyst
A technology for changing catalysts and catalysts, which is applied in chemical instruments and methods, inorganic chemistry, bulk chemical production, etc. It can solve the problems affecting the normal operation of the device, changing the condensate discharge and polluting the environment, and achieving the effect of high hydrogen production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Weigh 34.00g of zinc and 32.33g of copper respectively, add 171.6g of nitric acid, control the reaction temperature at 80-90°C, and prepare a mixed solution of copper nitrate and zinc nitrate with a certain concentration.
Embodiment 2
[0021] In the mixed solution in embodiment 1, add K 2 ZnO 2 solution, and then add saturated sodium carbonate solution dropwise to neutralize the precipitates of basic copper carbonate, basic zinc carbonate and basic potassium zinc carbonate. After stirring, and then filtering and drying, the mixed material of basic copper carbonate, basic zinc carbonate, basic potassium carbonate and aluminum hydroxide is prepared.
[0022] The dried mixed material is mixed with an appropriate amount of wet filter cake, rolled and mixed evenly, and then granulated and air-dried to remove part of the water. The corresponding oxides are prepared by roasting the dried particles at a high temperature of 450-520°C.
[0023] The oxide obtained by roasting is mixed with 1.33g of graphite, and then formed into a tablet to obtain the finished product.
Embodiment 3
[0025] In the mixed solution in embodiment 1, add Rb 2 ZnO 2 solution, and then add saturated sodium carbonate solution dropwise to neutralize the precipitates of basic copper carbonate, basic zinc carbonate and basic potassium zinc carbonate. Stir, and then filter and dry to prepare basic copper carbonate, basic zinc carbonate, basic rubidium carbonate and aluminum hydroxide mixed material.
[0026] The dried mixed material is mixed with an appropriate amount of wet filter cake, rolled and mixed evenly, and then granulated and air-dried to remove part of the water. The corresponding oxides are prepared by roasting the dried particles at a high temperature of 450-520°C.
[0027] The oxide obtained by roasting is mixed with 1.33g of graphite, and then formed into a tablet to obtain the finished product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com