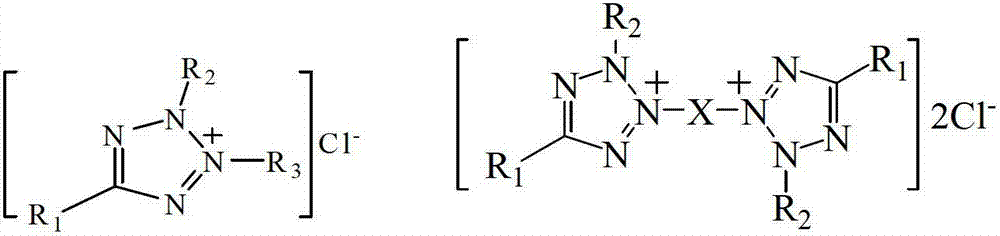
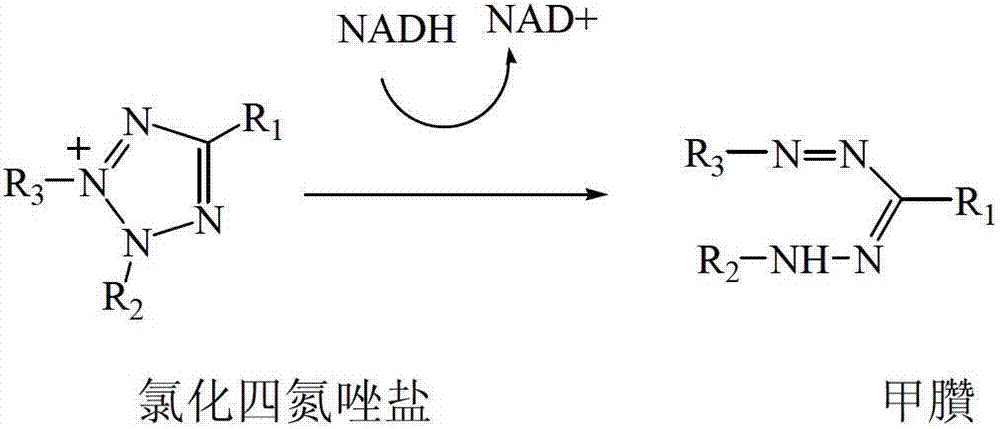
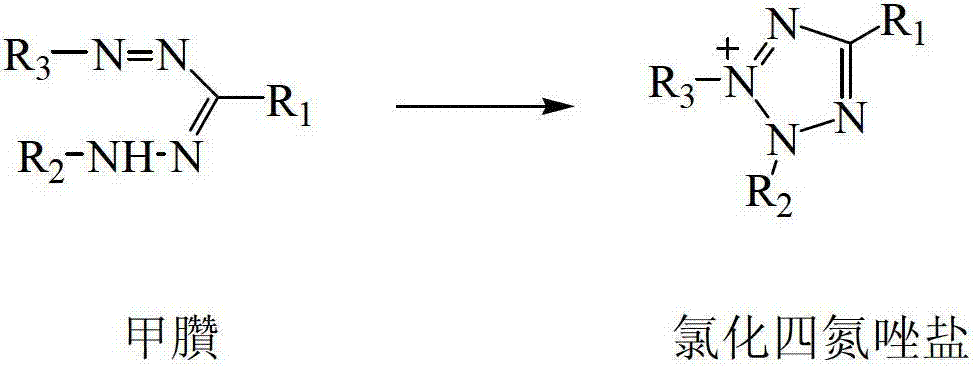
Preparation method of biochemical detection reagent of chlorinated tetrazolium salts
A technology of tetrazolium chloride salt and biochemical detection, applied in the direction of organic chemistry, can solve the problems of difficult processing, low yield, and many by-products, and achieve the effect of overcoming many by-products, high yield, and simple processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Preparation of 2-p-iodophenyl-3-p-nitrophenyl-5-phenyltetrazolium chloride
[0032]
[0033] Put 4.80g of formazan in a 150ml four-neck flask, add 50ml of methanol, then add 2.5ml of concentrated hydrochloric acid, stir to dissolve, and cool to 0°C. In the range of 0°C to 10°C, add 5ml of 30% hydrogen peroxide solution dropwise, after the dropwise addition, stir at room temperature for 3 hours, remove methanol by rotary evaporator, then extract with chloroform (3×30ml), dry over anhydrous magnesium sulfate After that, 30ml of anhydrous diethyl ether was added dropwise to precipitate a crude product, which was then recrystallized with methanol / diethyl ether (1:2), filtered, and vacuum-dried at 25°C for 24 hours to obtain 4.50 g of tetrazolium chloride salt crystals with a yield of 91%.
Embodiment 2
[0035] Preparation of 2,5-diphenyl-3-(1-naphthyl)tetrazolium chloride
[0036]
[0037] Put 3.51g of formazan in a 150ml four-neck flask, add 50ml of methanol, and then add 2.5ml of concentrated hydrochloric acid, stir to dissolve, and cool to 0°C. In the range of 0°C to 10°C, add 5ml of 30% hydrogen peroxide solution dropwise, after the dropwise addition, stir at room temperature for 5 hours, remove methanol by rotary evaporation, then extract with chloroform (3×30ml), dry over anhydrous magnesium sulfate , dropwise added 30ml of anhydrous diethyl ether to precipitate a crude product, then recrystallized the crude product with methanol / diethyl ether (1:2), filtered, and vacuum-dried at 25°C for 24 hours to obtain 3.55 g of tetrazolium chloride salt crystals, with a yield of 93 %.
Embodiment 3
[0039] Preparation of 3,3'-dimethoxy-4,4'-diphenylene-3",3"'-bis(2-p-nitrophenyl-5-phenyltetrazolium chloride)
[0040]
[0041] Put 8.17g of formazan into a 250ml four-necked flask, add 100ml of methanol, and then add 2.5ml of concentrated hydrochloric acid, stir to dissolve, and cool to 0°C. In the range of 0°C to 10°C, add 5ml of 30% hydrogen peroxide solution dropwise, after the dropwise addition, stir at room temperature for 8 hours, remove methanol by rotary evaporation, then extract with chloroform (3×50ml), dry over anhydrous magnesium sulfate , dropwise added 60ml of anhydrous diethyl ether to precipitate a crude product, then recrystallized the crude product with methanol / diethyl ether (1:1.5), filtered, and vacuum-dried at 25°C for 24 hours to obtain 7.11 g of tetrazolium chloride salt crystals, with a yield of 87 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com