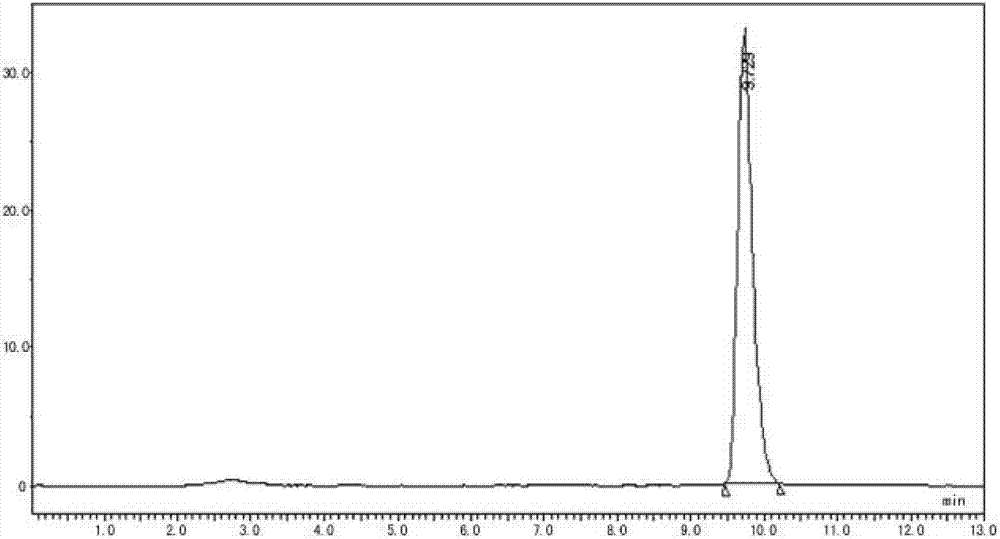
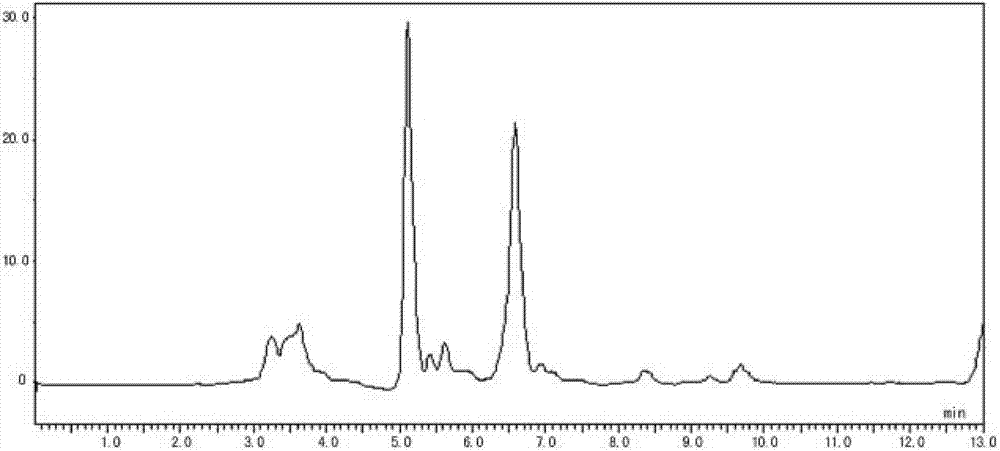
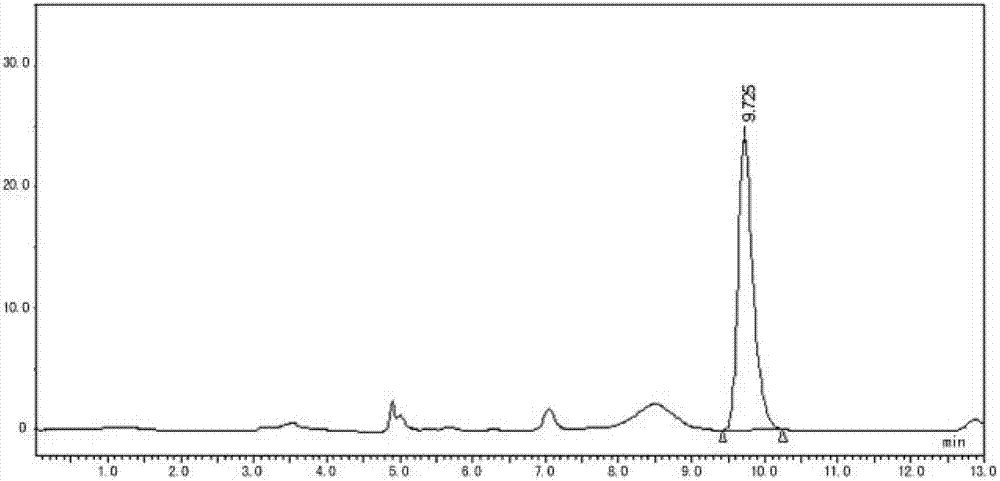
Enrofloxacin injection and preparation method thereof
A technology of enrofloxacin and injection, which is applied in the field of enrofloxacin injection and its preparation, can solve the problems of restricting the scope of clinical application of enrofloxacin injection, unfavorable clinical drug compatibility, low concentration of enrofloxacin, etc. problem, to achieve the effect of reducing single administration dose, simple and feasible preparation process, and reducing irritation and injury
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0057] The preparation method of above-mentioned enrofloxacin injection, the steps are as follows:
[0058] (1) Weigh the water for injection for dissolving various medicaments;
[0059] ⑵Add antioxidant and stir until completely dissolved;
[0060] (3) Add the main ingredient and stir evenly;
[0061] (4) Add co-solvent and stir until the solution is clear;
[0062] (5) Add the remaining water for injection to the full amount, filter, subpackage, and sterilize to obtain enrofloxacin injection.
Embodiment 1
[0064] A kind of enrofloxacin injection, its composition and parts by weight are as follows:
[0065] Enrofloxacin 21g;
[0066] Lactic acid 5g;
[0068] Water for injection 73.9g.
[0069] The preparation method of above-mentioned enrofloxacin injection, the steps are as follows:
[0070] (1) Weigh 50g of water for injection;
[0071] (2) Add 0.1 g of sodium bisulfite to the solution in step (1), and stir until completely dissolved;
[0072] (3) Add 21 g of enrofloxacin to the solution in step (2), and stir evenly;
[0073] (4) Add 5 g of lactic acid to the step (3) solution, and stir until the solution is clarified;
[0074] (5) Add the remaining water for injection to the solution in step (4) to 100 g, filter, subpackage, and sterilize to obtain enrofloxacin injection.
Embodiment 2
[0076] A kind of enrofloxacin injection, its composition and parts by weight are as follows:
[0077] Enrofloxacin 22g;
[0078] Lactic acid 8g;
[0080] Water for injection 69.8g.
[0081] The preparation method of above-mentioned enrofloxacin injection, the steps are as follows:
[0082] (1) Weigh 50g of water for injection;
[0083] (2) Add 0.2 g of sodium bisulfite to the solution in step (1), and stir until completely dissolved;
[0084] (3) Add 22 g of enrofloxacin to the solution in step (2), and stir evenly;
[0085] (4) Add 8 g of lactic acid to the step (3) solution, and stir until the solution is clarified;
[0086] (5) Add the remaining water for injection to the solution in step (4) to 100 g, filter, subpackage, and sterilize to obtain enrofloxacin injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com