Bifunctional basic ionic liquid and water-phase catalytic synthesis of substituted pyridinium compound by using same
A basic ion, bifunctional technology, applied in the field of bifunctional basic ionic liquid and its aqueous phase catalytic synthesis of substituted pyridine compounds, can solve the problems of no large-scale industrial application, complicated operation process, many side reactions, etc. Achieve the effect of being conducive to large-scale industrial production, wide source of raw materials, and convenient preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
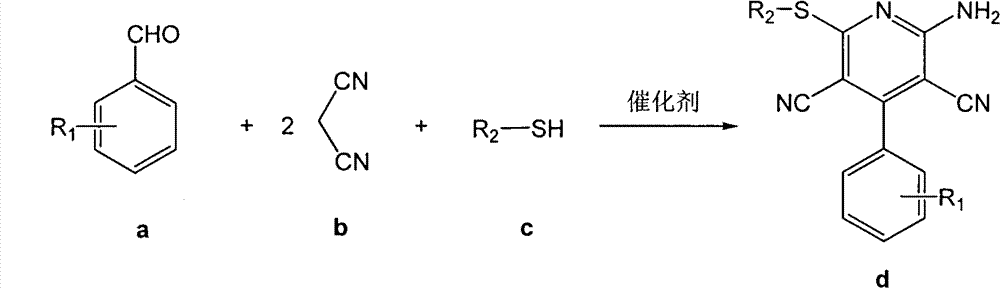
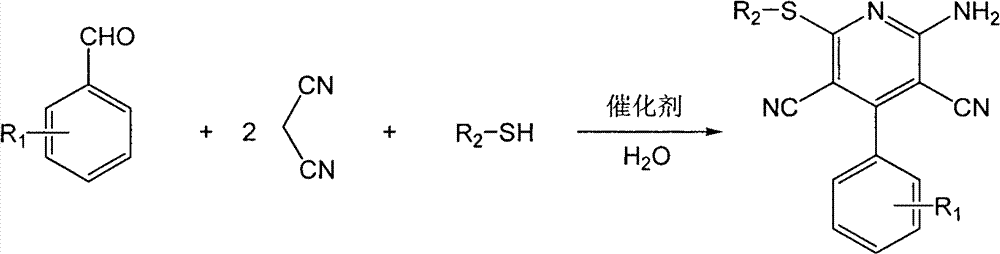
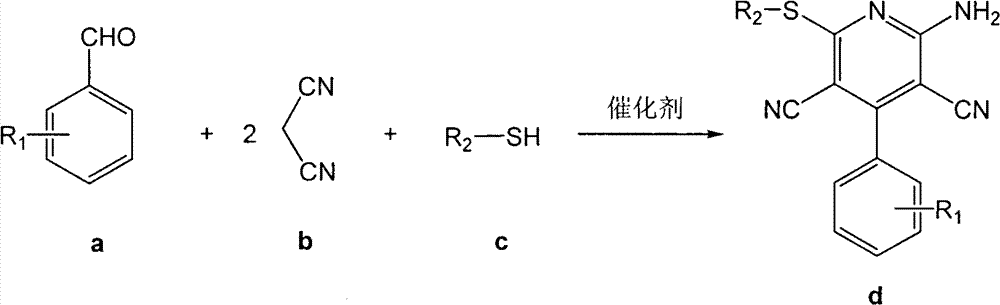
[0025] In a 25mL round-bottomed flask, add 10mmol (1.061g) benzaldehyde, 20mmol (1.321g) propanedinitrile, 10mmol (0.6213g) ethanethiol, 1.5mmol bifunctional basic ionic liquid, 4mL water successively, at room temperature , mixed and stirred under normal pressure for 2.5 hours, filtered and washed with cold water, recrystallized from 95% ethanol to obtain the pure product of 6-ethylthio-2-amino-4-phenyl-3,5-dicyanopyridine, the yield 75%.
Embodiment 2
[0027] In a 25mL round bottom flask, add 10mmol (1.061g) benzaldehyde, 20mmol (1.321g) propanedinitrile, 10mmol (0.781g) 2-mercaptoethanol, 0.5mmol bifunctional alkaline ionic liquid, 5mL water in sequence, Mix and stir under normal pressure at 60°C for 0.5 hours, filter and wash with cold water, and recrystallize from 95% ethanol to obtain the pure product of 6-hydroxyethylthio-2-amino-4-phenyl-3,5-dicyanopyridine , the yield was 75%.
Embodiment 3
[0029] In a 25mL round-bottomed flask, add 10mmol (1.061g) benzaldehyde, 20mmol (1.321g) propanedinitrile, 10mmol (1.102g) thiophenol, 1.0mmol bifunctional basic ionic liquid, 10mL water, at room temperature , mixed and stirred under normal pressure for 1.0 hour, filtered and washed with cold water, recrystallized from 95% ethanol to obtain the pure product of 6-phenylsulfanyl-2-amino-4-phenyl-3,5-dicyanopyridine, the product The rate is 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com