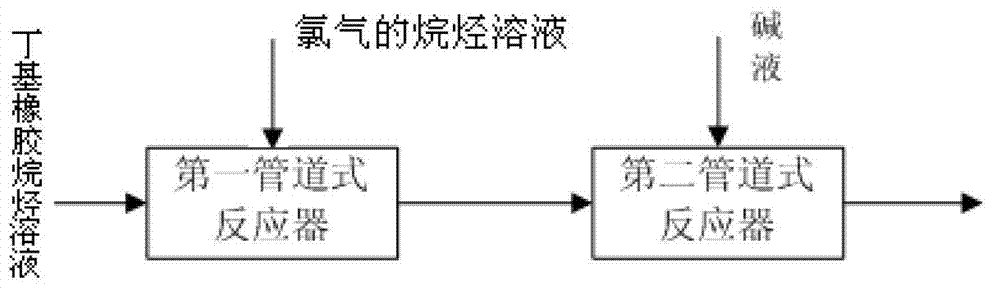
Chlorinated butyl rubber synthesizing process
A chlorinated butyl rubber and synthesis process technology, which is applied in the field of chemical synthesis, can solve problems such as high energy consumption, difficult product quality, and product processability decline, and achieve low energy consumption, simple equipment, and reduced occurrence of transposition side reactions The effect of the probability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] according to figure 1 The flow chart of the butyl rubber n-hexane solution with a concentration of 25wt% and a concentration of 10wt% Cl 2 The normal hexane solution and lye are 10wt% sodium hydroxide aqueous solution as raw materials; wherein the unsaturation of butyl rubber is 1.9%, Cl 2 Cl in n-hexane solution 2 The molar ratio to the double bond in butyl rubber is 0.7, NaOH and Cl 2 The molar ratio is 1:1. The temperature of each feed is 30oC; The flow velocity in the follow-up pipeline of the first pipeline reactor is 0.3m / s, and the residence time is 60 seconds; The flow velocity in the follow-up pipeline of the second pipeline reactor is 0.3m / s, and the residence time is 300 seconds. The product chlorinated butyl rubber has a chlorination degree of 2%, a secondary chlorine content of 70%, an unsaturation of 0.5%, a rubber number average molecular weight of 180,000 Daltons, and a molecular weight distribution PDI of 2.5.
Embodiment 2
[0020] according to figure 1 The flow chart of the butyl rubber n-heptane solution with a concentration of 5wt% and a concentration of 8wt% Cl 2 The n-heptane solution and the 2wt% sodium hydroxide aqueous solution as the lye are raw materials. Butyl rubber has an unsaturation of 1.2%. Cl 2 Cl in n-heptane solution 2 The molar ratio to the double bond in butyl rubber is 1.1, NaOH and Cl 2 The molar ratio is 2:1. The temperature of each feed is 20oC; the flow velocity in the follow-up pipeline of the first pipeline reactor is 1m / s, and the residence time is 10 seconds; the flow velocity in the follow-up pipeline of the second pipeline reactor is 1m / s, and the residence time is 60 seconds . The product chlorinated butyl rubber has a chlorination degree of 0.8%, a secondary chlorine content of 99%, a degree of unsaturation of 0.5%, a rubber number average molecular weight of 230,000 Daltons, and a molecular weight distribution PDI of 1.9.
Embodiment 3
[0022] according to figure 1 The flow chart of the butyl rubber n-octane solution with a concentration of 5wt% and a concentration of 2wt% Cl 2 The n-octane solution and the 0.1wt% sodium hydroxide aqueous solution as the lye are raw materials. Butyl rubber has an unsaturation of 3.0%. Cl 2 Cl in n-octane solution 2 The molar ratio of the double bonds in the raw material butyl rubber is 2.0, NaOH and Cl 2 The molar ratio is 1.5:1. The temperature of each feed is 50oC; the flow velocity in the follow-up pipeline of the first pipeline reactor is 2m / s, and the residence time is 6 seconds; the flow velocity in the follow-up pipeline of the second pipeline reactor is 2m / s, and the residence time is 20 seconds . The product chlorinated butyl rubber has a chlorination degree of 1.2%, a secondary chlorine content of 99%, a degree of unsaturation of 1.2%, a rubber number average molecular weight of 220,000 Daltons, and a molecular weight distribution PDI of 2.3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com