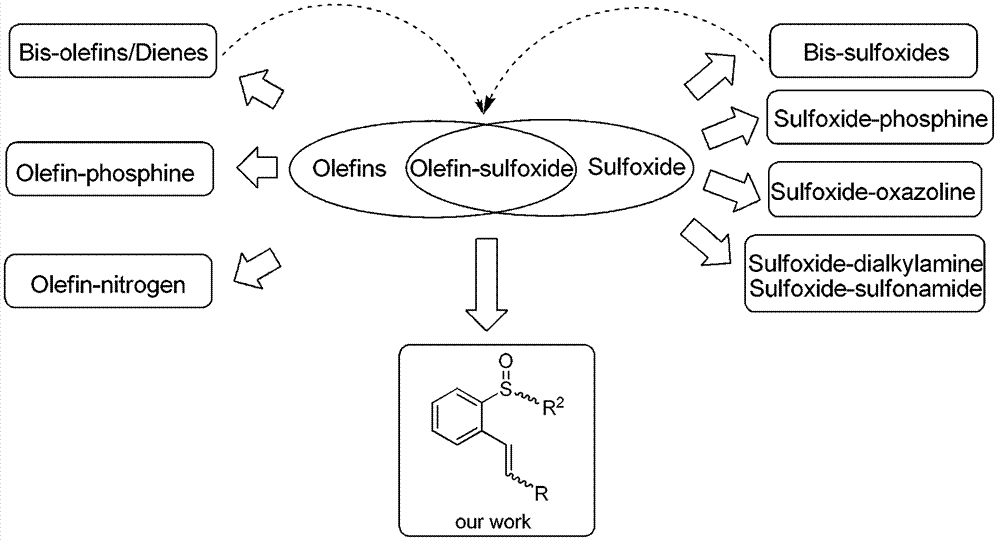
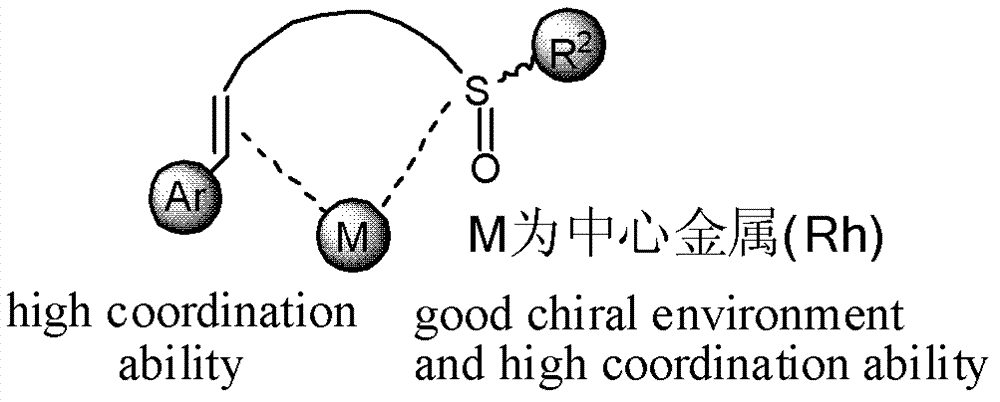
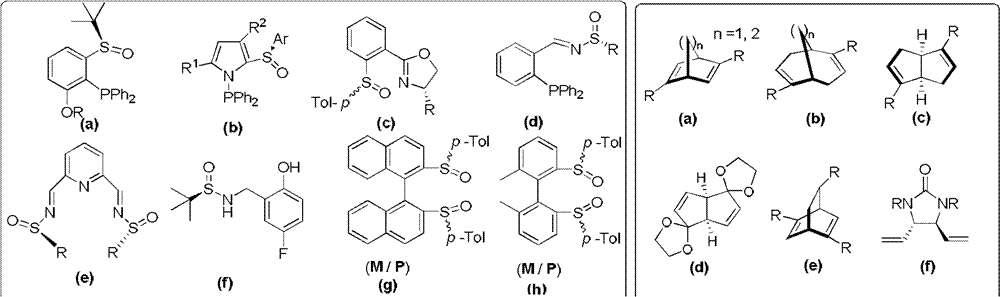
Chiral sulfoxide alkene ligand, preparation method and application thereof
A sulfoxide and ligand technology, which is applied to chiral sulfoxide ligands and the fields of preparation and application thereof, can solve problems such as no literature reports, and achieve the effects of easy storage, mild synthesis conditions and short synthesis routes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] Taking ligand 1c in Formula 1 as an example, the synthetic route is as in Formula 7:
[0025]
[0026] The synthetic route of formula 7 chiral sulfoxide ene ligand 1c
[0027] Scheme 1.Synthesis of olefin-sulfoxide ligand 1c.Reagents and conditions: a) P(OEt)3, 160℃, 5h; b) NaH, THF, 0℃to room temperature, 1h; then4-fluorobenzaldehyde, THF, 0℃ to room temperature, overnight, 89%; c) n-BuLi, THF, -78°C; then(R)-thiosulfinate, THF, -78°C to room temperature, overnight, 52.3%.
[0028] The specific operation steps are as follows:
[0029] a): With reference to the literature [Blake, Alexander J.; Harding, Mervyn; Sharp, John T.;. ) into 0.14 mol of triethyl phosphite, slowly heated to 160°C, heated for 5 hours, cooled to room temperature, and slowly distilled under reduced pressure to obtain 33.6g of colorless oily liquid (130-132°C, 1mmHg). Yield 92%.
[0030] b): Under argon atmosphere, put 1mmol o-bromobenzyl phosphite diethyl (B) into 2ml of THF, slowly add 1.2m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com