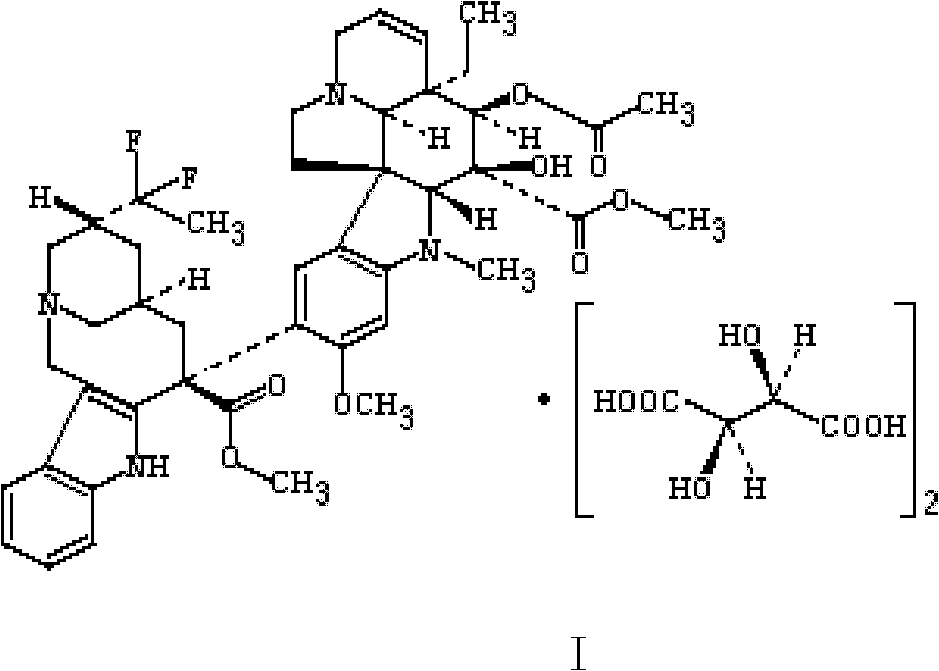
Vinflunine liposome preparation and preparation method of vinflunine liposome preparation
A technology of liposome preparation, Changchun Flunine, which is applied in the direction of liposome delivery, pharmaceutical formulation, medical preparations of non-active ingredients, etc., can solve the problems of increased toxicity, poor targeting, leakage, etc., and achieve reduction Vascular irritation and phlebitis, prolong half-life in vivo, and avoid phagocytosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
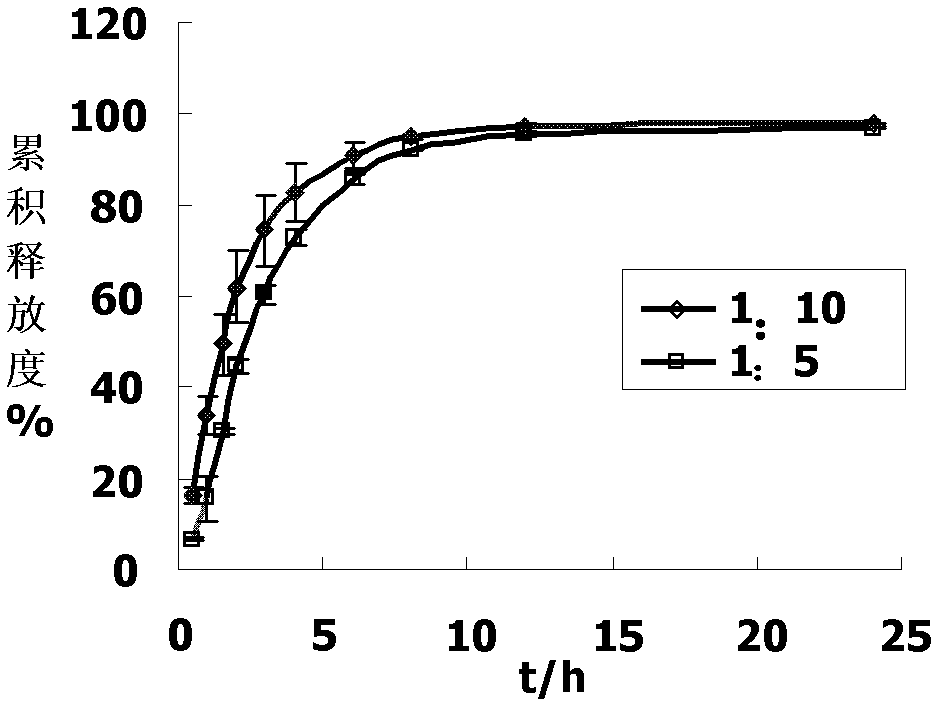
[0072] Polydispersity Index (PDI): It reflects the distribution of liposome particle size, and the smaller the value, the more concentrated the particle size distribution. Embodiment 1, passive drug loading method prepares Vinflunine liposome (comparative example)
[0073] The liposome membrane materials for encapsulating vinflunine are all: hydrogenated soybean lecithin, cholesterol and PEG-DSPE, with a mass ratio of 1:0.2:0.1.
[0074] Thin film dispersion method
[0075] Dissolve the liposome membrane material in an appropriate amount of ethanol, remove the ethanol under reduced pressure to form a dry film, add vinflunine bitartrate saline solution (4 mg / ml) to hydrate the phospholipid membrane, and obtain liposomes. The particle size is reduced to about 200nm by microfluidics.
[0076] reverse phase evaporation
[0077] Appropriate amount of dichloromethane-ether (1:1 V / V) dissolves the membrane material, adds vinflunine bitartrate normal saline solution (4mg / ml), condu...
Embodiment 2
[0081] Embodiment 2, ammonium sulfate gradient method prepares vinflunine liposome
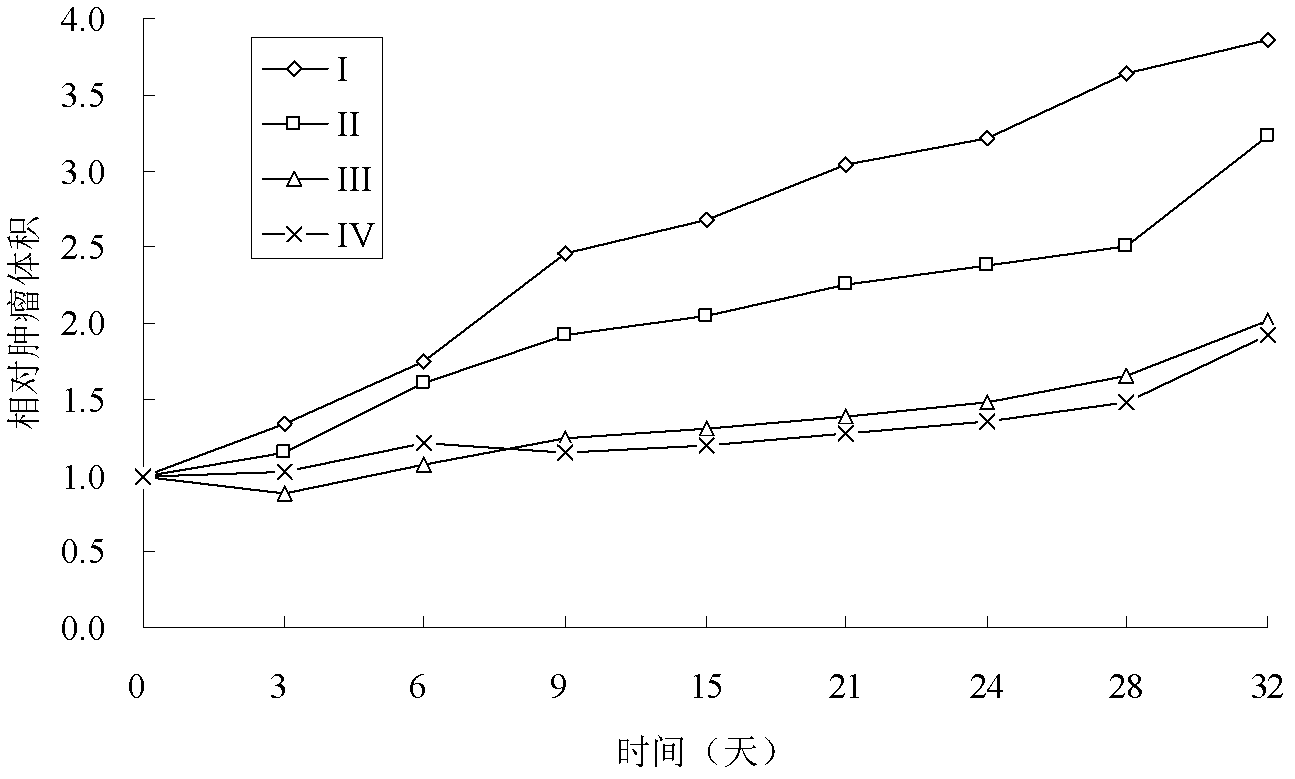
[0082] The liposome membrane material is hydrogenated soybean phospholipid, cholesterol and PEG-DSPE, the mass ratio is 1:0.2:0.1, dissolved in an appropriate amount of ethanol, and 300mmol L of ammonium sulfate solution is added -1 , hydrated in a water bath at 60°C for 40 minutes, treated with a high-pressure homogenizer to reduce the particle size to about 200nm, then sterilized by filtration with a 0.22μm microporous membrane, and dialyzed to remove ammonium sulfate in the outer aqueous phase to obtain ammonium sulfate gradient liposomes. Add vinflunine bitartrate normal saline solution into this gradient liposome, keep the drug loaded in a water bath at 60°C for 10 minutes, and obtain vinflunine liposome after sterilization and filtration, wherein the content of vinflunine bitartrate is 2 mg / ml. After removing the free drug in the outer aqueous phase, the encapsulation efficiency was de...
Embodiment 3
[0083] Embodiment 3 pH gradient method prepares vinflunine liposome
[0084] The liposome membrane material is hydrogenated soybean phospholipid, cholesterol and PEG-DSPE, the mass ratio is 1:0.2:0.1, dissolved in an appropriate amount of ethanol, and 300mmol L of citrate buffer is added -1 , pH 4.0, hydrated in a water bath at 65°C for 20 minutes to obtain the primary product of blank liposomes; treated with a high-pressure homogenizer to reduce the particle size to 150-200 nm, and then sterilized with a 0.22 μm microporous membrane to obtain blank liposomes .
[0085] Take blank liposome suspension 10.0mL, add 500mmol·L -1 Sodium phosphate solution 6mL, mix, then add 4mg·mL -1 5 mL of vinflunine bitartrate normal saline solution was incubated at 60° C. for 10 minutes, and liposomes were prepared after sterilizing and filtering. The encapsulation efficiency was determined to be 95.4% by gel column method.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com