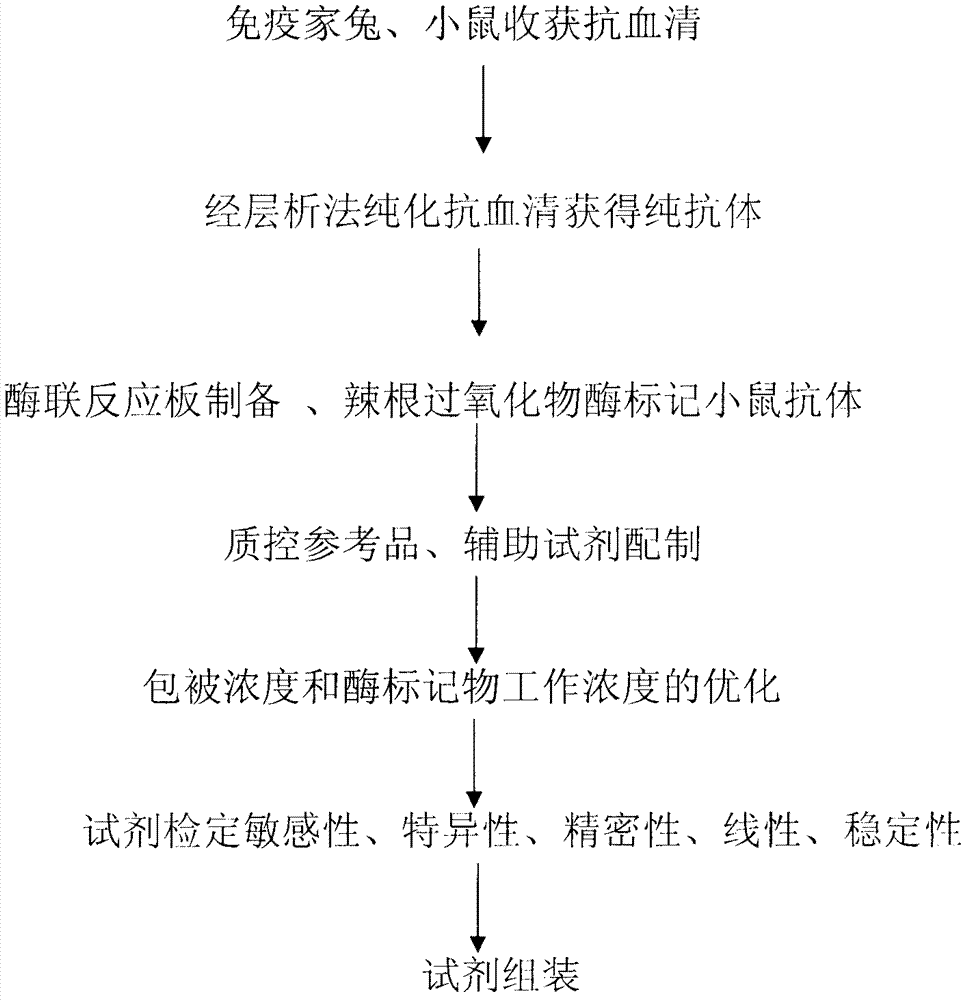
Preparation and application of ELISA (Enzyme-Linked Immunosorbent Assay) kit for detecting novel bunyavirus antigen
A new bunya virus and virus antigen technology, applied in the field of immunodiagnosis in the field of biotechnology, can solve problems such as lack of training in differential diagnosis for primary medical practitioners, lack of verification methods for virus infection, and inability to assess the protective effect of vaccines on previous infection levels.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1: New bunya virus (JS-2007-001 virus strain) immunization rabbit and serum antibody preparation
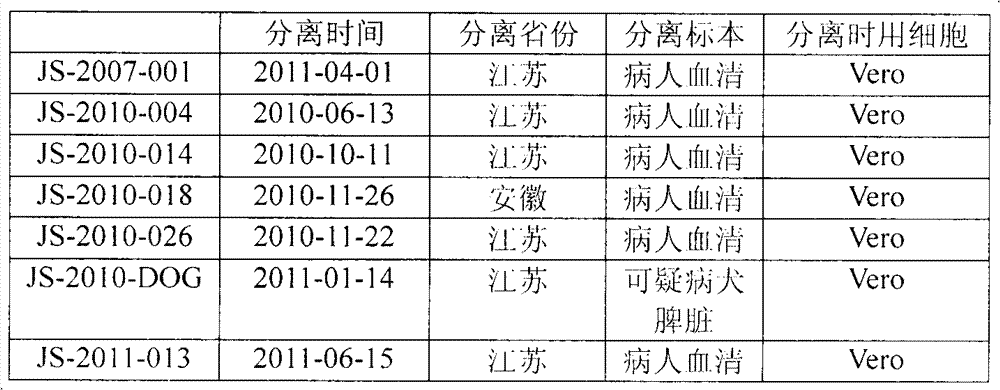
[0017] Seven New Bunia virus strains were isolated from Jiangsu and Anhui epidemic areas, 6 strains were directly isolated from patient serum, and 1 strain was from a suspected sick dog. All cells were VERO cells when isolated (Table 1).
[0018] Table 1: Isolation time and source of 7 New Bunyavirus strains
[0019]
[0020] Adaptive culture, immunogenicity identification and immunoprotection identification of 7 New Bunia virus strains in VERO cells found that the JS-2007-001 virus strain has the best growth characteristics and adaptability characteristics. When the growth peak is reached, a higher titer virus can be obtained stably, and the antibody level of the immunized animals is higher. The immune serum neutralization test found that it has a good protective effect on all 7 strains of the virus. So choose the JS-2007-001 virus seed as the prepared vir...
Embodiment 2
[0024] Example 2: New Bunia virus (JS-2007-001 strain) immunization of mice and serum antibody preparation and HRP labeling
[0025] Dilute the purified and inactivated Neobunia virus antigen with normal saline in an appropriate amount to a content of 0.1-200 μg / mL, add Freund's complete adjuvant (Sigma) for mixing, select 15-25 g mice, and then Inject 1 to 5 mg of BCG in the legs for sensitization. Two weeks later, the mice were intraperitoneally injected with the above-mentioned adjuvant antigen, and boosted with the same dose of different immunized parts at intervals of 2 to 4 weeks. A total of 3 to 5 immunizations were carried out. The mouse serum was collected 10 days after the last immunization for antibody For titer determination, whole blood was collected after reaching a ratio greater than 1:20000, and serum was routinely separated. Pure antibody was purified by Protein G conventional method.
[0026] Preparation of HRP-labeled mouse anti-New Bunia virus antibody. ...
Embodiment 3
[0027] Example 3: Antibody-coated plate preparation.
[0028] The purified antibody was appropriately diluted (1-10 μg / mL) with 0.05M pH 9 carbonate coating buffer. Add the diluted antibody solution into the wells of the enzyme-linked plate, 100 μL per well, and coat at 4°C for 18-20 hours, discard the liquid in the wells, wash the plate several times, and add enzyme stabilizer (Shandong, Taitianhe Bio) In each plate well, 150-200 μL per well. React at 4°C for 4-6 hours. Discard the protective agent in the well, dry the coated plate by freeze-drying method, seal the enzyme-linked plate and store it at 2-8°C for later use.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com