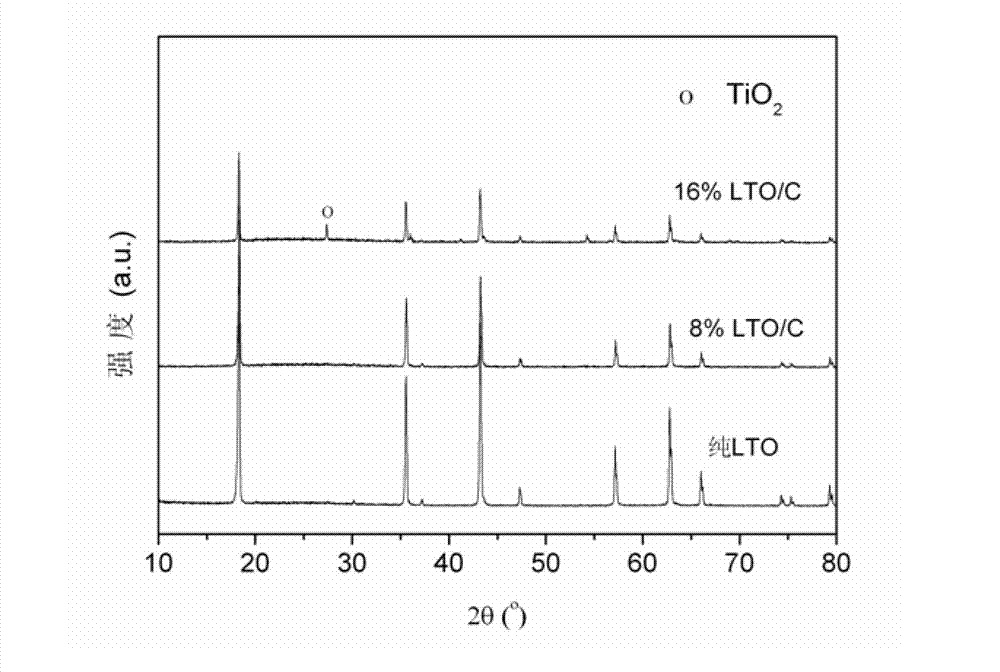
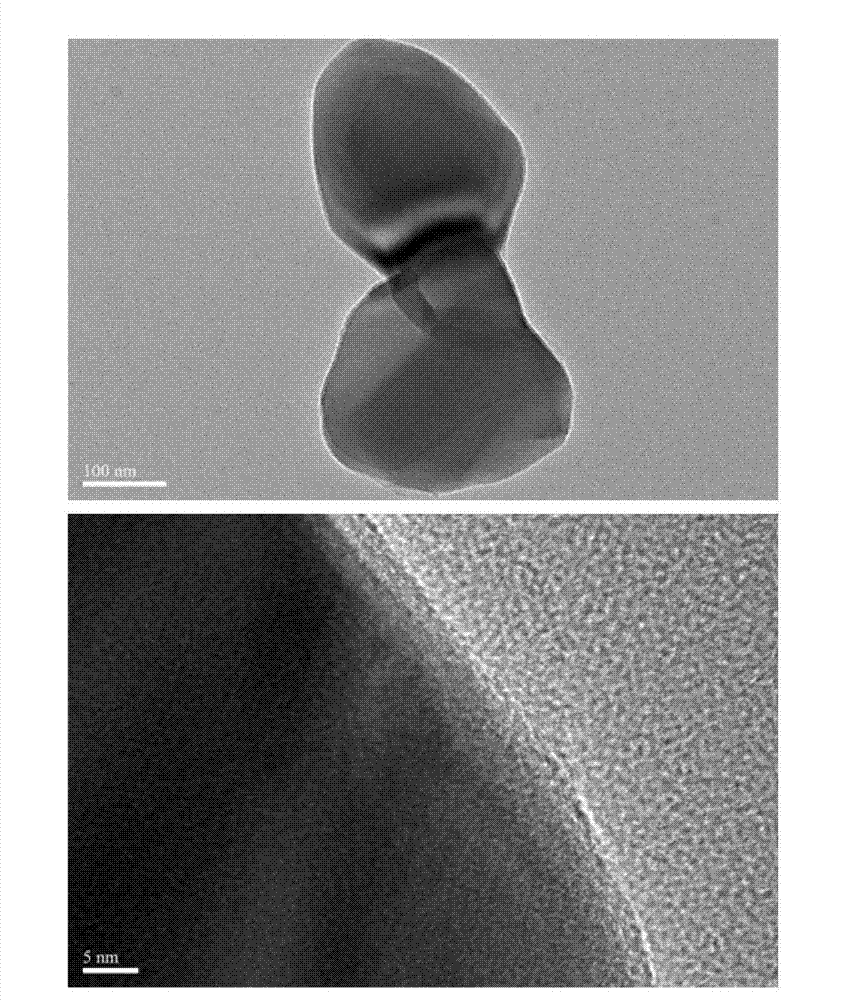
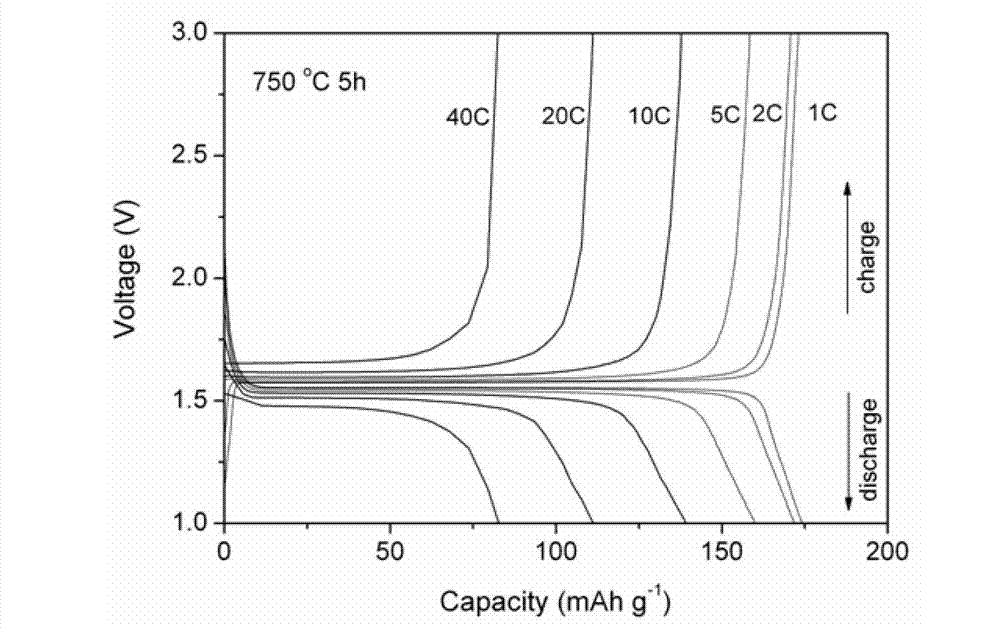
Method for preparing carbon-coated nano-lithium titanate by ethylene diamine tetraacetic acid-citric acid (EDTA-CA) joint complexation
A nano-lithium titanate and carbon coating technology, applied in the field of nano-lithium titanate, can solve the problems of affecting battery performance, easy enrichment of electrons, and poor conductivity, so as to improve mixing uniformity, alleviate particle agglomeration, and improve electrical conductivity sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Add 3.4mL of HNO to 170.16mL of absolute ethanol 3As a hydrolysis inhibitor of tetrabutyl titanate, solution A was obtained; according to the molar ratio Li:Ti=4.2:5, 8.508g tetra-n-butyl titanate (analytical pure) and 0.776g lithium carbonate ( Analytical pure, pre-ground into fine powder), magnetically stirred until completely dissolved and clear; according to the molar ratio of EDTA:CA=1:2, weigh 13.443g EDTA and 17.677gCA, drop 10mL distilled water for pre-mixing, then add 35.4mL ammonia water dissolved to obtain solution B. Mix solution A and solution B, adjust the pH of the mixed solution to 10 with ammonia water, and continue stirring until the mixed solution becomes clear as a sol. Continue heating and stirring at 100°C for 3 hours. After the sol becomes a transparent gel, put it in a blast oven at 240°C and dry it for 12 hours to obtain a black fluffy Li 4 Ti 5 o 12 Precursor. The precursor is ground and put into a muffle furnace, and calcined a...
Embodiment 2
[0031] Example 2: Mix 85.08mL of absolute ethanol with 8.51mL of deionized water according to the volume ratio of 1:0.1, add 8.51mL of hydrochloric acid as the hydrolysis inhibitor of the subsequent reactant, and obtain solution A; according to the molar ratio Li:Ti= 4.3:5, put 8.508g tetra-n-butyl titanate (analytical pure) and 1.419g lithium acetate (analytical pure) into solution A, stir magnetically until completely dissolved and become clear; according to the molar ratio, EDTA:CA=1: 1.5. Weigh 6.795g EDTA and 6.701g CA, drop 5mL of distilled water for pre-mixing, then add 15.21mL of ammonia water to dissolve, and obtain solution B. Mix solution A and solution B, adjust the pH of the mixed solution to 7 with ammonia water, and continue stirring until the mixed solution becomes clear as a sol. Continue heating and stirring at 80°C for 4 hours. After the sol becomes a transparent gel, put it in a blast oven at 240°C and dry it for 12 hours to obtain a black fluffy Li 4 Ti ...
Embodiment 3
[0032] Example 3: Mix 112.18mL of absolute ethanol with 22.44mL of deionized water according to the volume ratio of 1:0.2, add 22.44mL of acetic acid as the hydrolysis inhibitor of the subsequent reactant, and obtain solution A; according to the molar ratio Li:Ti= 4.0:5, put 7.105g tetraisopropyl titanate (analytical pure) and 1.38g lithium nitrate (analytical pure) into solution A, stir magnetically until completely dissolved and become clear; according to the molar ratio, EDTA:CA=1: 1. Weigh 13.151g of EDTA and 8.646g of CA, add 10mL of distilled water to pre-mix, then add 24.24mL of ammonia water to dissolve, and obtain solution B. Mix solution A and solution B, adjust the pH of the mixed solution to 8 with ammonia water, and continue stirring until the mixed solution becomes clear as a sol. Continue heating and stirring at 60°C for 5 hours. After the sol becomes a transparent gel, put it in a blast oven at 240°C and dry it for 12 hours to obtain a black fluffy Li 4 Ti 5 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Discharge capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com