Preparation method of Mn-Ti oxide system low-temperature selective catalytic reduction (SCR) catalyst
A selective and oxidizing technology, applied in the field of physical chemistry, can solve the problems of increasing denitration costs, shortening catalyst life, increasing investment costs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] A kind of preparation method of Mn-Ti oxide system low-temperature selective catalytic reduction catalyst, its specific steps are as follows:
[0019] (1) Under stirring conditions, add n-butyl titanate to acrylic acid (AA) and mix well; then add chemically pure manganese nitrate, wherein the molar ratio of n-butyl titanate, manganese nitrate and acrylic acid is 10:3 : 26; Then add ethanol, stir to form a homogeneous solution;
[0020] (2) Nitrogen is passed to remove the oxygen in the solution system, and then (NH 4 ) 2 S 2 o 8 The initiator, after mixing evenly, is placed in an oven to polymerize at a temperature of 80°C to obtain a solid polyacrylate;
[0021] (3) Dry the solid polyacrylate at 200° C. for 24 hours to obtain a polymer precursor.
[0022] (4) After the polymer precursor was pyrolyzed at 800 °C for 5 hours in an air atmosphere, it was ground to obtain the low-temperature selective catalytic reduction catalyst of the Mn-Ti oxide system, that is, the...
Embodiment 2
[0024] A kind of preparation method of Mn-Ti oxide system low-temperature selective catalytic reduction catalyst, its specific steps are as follows:
[0025] (1) Under stirring conditions, add n-butyl titanate to acrylic acid (AA) and mix well; then add chemically pure manganese nitrate, wherein the molar ratio of n-butyl titanate, manganese nitrate and acrylic acid is 5:2 : 21; Then add ethanol and water, stir to form a homogeneous solution;
[0026] (2) Nitrogen is passed to remove the oxygen in the solution system, and then (NH 4 ) 2 S 2 o 8 The initiator, after mixing evenly, is placed in an oven to polymerize at a temperature of 80°C to obtain a solid polyacrylate;
[0027] (3) Dry the solid polyacrylate at 220° C. for 24 hours to obtain a polymer precursor.
[0028] (4) The polymer precursor was pyrolyzed at 700°C for 6 hours in an air atmosphere, and then ground to obtain Mn-Ti oxide powder.
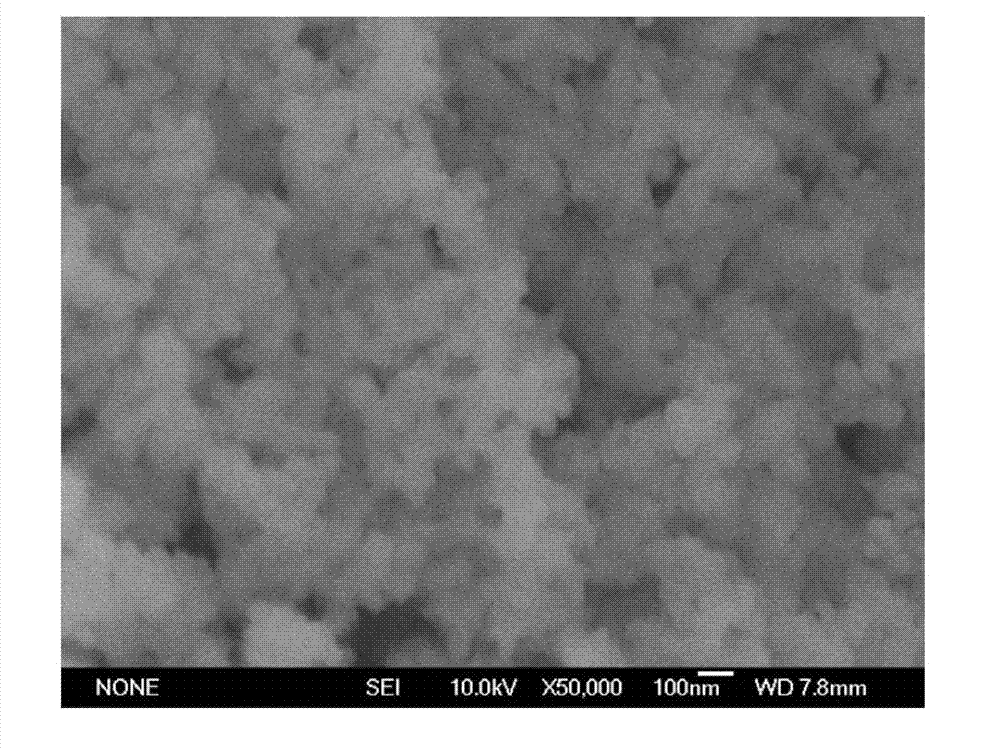
[0029] From the scanning electron micrograph of the final Mn-Ti oxide p...
Embodiment 3
[0031] A kind of preparation method of Mn-Ti oxide system low-temperature selective catalytic reduction catalyst, its specific steps are as follows:
[0032] (1) Under stirring conditions, add n-butyl titanate to acrylic acid (AA) and mix well; then add chemically pure manganese nitrate, wherein the molar ratio of n-butyl titanate, manganese nitrate and acrylic acid is 2:1 : 12; Then add Virahol and water, stir to form a homogeneous solution;
[0033] (2) Nitrogen is passed to remove the oxygen in the solution system, and then (NH 4 ) 2 S 2 o 8 The initiator, after mixing evenly, is placed in an oven to polymerize at a temperature of 80°C to obtain a solid polyacrylate;
[0034] (3) Dry the solid polyacrylate at 240° C. for 36 hours to obtain a polymer precursor.
[0035] (4) The polymer precursor was pyrolyzed at 600°C for 8 hours in an air atmosphere and ground to obtain Mn-Ti oxide powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com