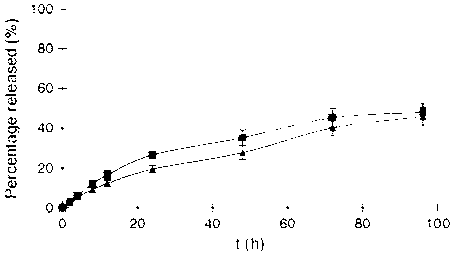
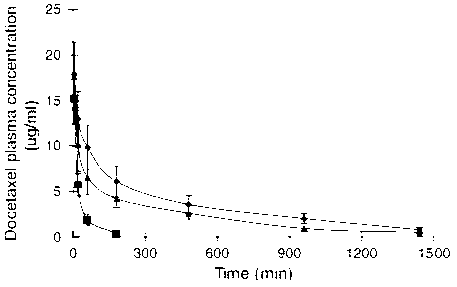
Docetaxel transferrin acceptor-targeted liposome preparation
A technology of docetaxel and transferrin is applied in the field of docetaxel-transferrin receptor targeting liposome preparation, which can solve the problems of occurrence and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: Preparation of docetaxel-transferrin receptor-targeted liposome formulation
[0020] The steps are as follows: Weigh 13.56mg of hydrogenated soybean lecithin, 35.34mg of lecithin, 35.45mg of cholesterol, 5.3mg of polyethylene glycol-distearoylphosphatidylethanolamine, mix with docetaxel (5.67mg) and spin steam, add phosphoric acid Salt buffer (pH 7.4, the same below) hydrated multilamellar liposomes, using post-embedding technology to link transferrin ligands to the surface of liposomes loaded with docetaxel, freeze-dried to obtain the product.
[0021] The specific steps for linking the transferrin ligand to the surface of the liposome containing docetaxel by the post-application embedding technology are as follows (the following examples are all the same): the transferrin is thiolated, and the hydroxyethylpiperazine Add Traut's reagent to transferrin (M Tr :M Tf =10:1), the mixture was obtained after sufficient reaction, the mixture containing transferrin...
Embodiment 2
[0022] Example 2: Preparation of docetaxel-transferrin receptor-targeted liposome formulation
[0023] The steps are as follows: Weigh 14.66 mg of hydrogenated soybean lecithin, 46.75 mg of soybean lecithin, 55.34 mg of cholesterol, and 5.4 mg of synthetic phosphatidylserine, mix with docetaxel (5.64 mg) and spin steam, add phosphate buffer to hydrate the multilamellar lipid The body is obtained by linking the transferrin ligand to the surface of the liposome loaded with docetaxel by post-embedding technology, and freeze-drying.
Embodiment 3
[0024] Example 3: Preparation of docetaxel-transferrin receptor-targeted liposome formulation
[0025] The steps are as follows: Weigh 50.44 mg of hydrogenated soybean lecithin, 70.45 mg of lecithin, 70.89 mg of cholesterol, 5.44 mg of synthetic dipalmitoylphosphatidylcholine, mix with docetaxel (10.55 mg) and spin steam, add phosphate buffer to hydrate The multi-lamellar liposome is obtained by linking the transferrin ligand to the surface of the liposome loaded with docetaxel by post-embedding technology, and freeze-drying.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com