Polylactic acid segmented copolymer and modified polylactic acid preparation method
A technology of block copolymer and polylactic acid, which is applied in the field of preparation of polylactic acid block copolymer and modified polylactic acid, can solve the problems of low reaction efficiency, increased production cost, unstable samples, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The invention provides a method for preparing a polylactic acid block copolymer, comprising the following steps: A) reacting branched polylactic acid and a halogenated acid halide under the action of a first catalyst to obtain a macromolecular initiator, the halogenated acid halide The acyl halide is a halogenated isobutyryl halide and / or a halogenated propionyl halide; B) the macroinitiator is mixed with glycidyl methacrylate under the action of cuprous halide and N-containing ligands, in a solvent The reaction was carried out to obtain a polylactic acid block copolymer.
[0034] The branched polylactic acid described in the present invention is preferably prepared according to the following steps: react lactide and polyhydric alcohol under the action of the second catalyst by heating to obtain branched polylactic acid, the number of hydroxyl groups of the polyhydric alcohol is ≥3, preferably 3~20, more preferably 3~10.
Embodiment 1
[0061] A 500mL reaction bottle with two vents was vacuum-baked and degassed, and after repeated argon filling three times, 200g (1.4mol) lactide, 0.13g (1.4×10 -3 mol) glycerol with 0.28g (7×10 -4 mol) stannous octoate, sealed system, heated to 120°C, stirred and reacted for 24 hours to obtain a white crystalline polymer, dissolved in chloroform, precipitated with excess ethanol to obtain a white solid, dried in vacuum to constant weight, and obtained branched polylactic acid , the relative weight average molecular weight is 180.0kg / mol, the relative molecular weight distribution is 1.50, and the relative molecular weight distribution is a relatively narrow peak.
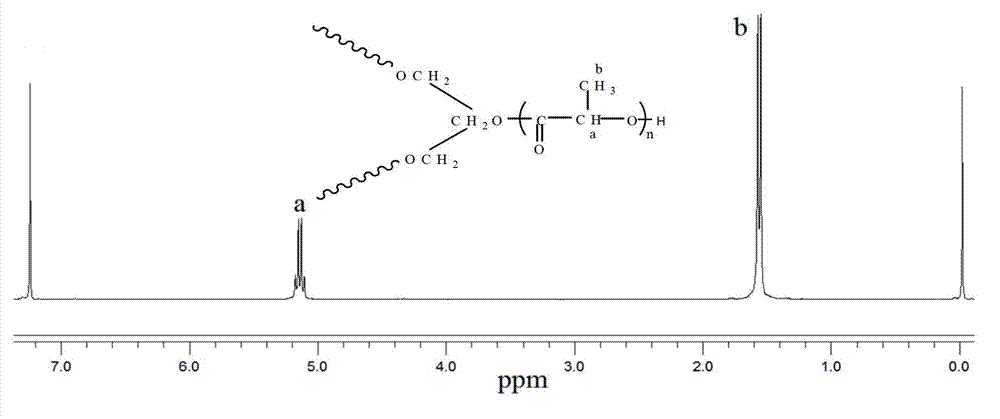
[0062] Utilize nuclear magnetic resonance to analyze the branched polylactic acid obtained in embodiment 1, obtain its hydrogen nuclear magnetic resonance spectrum figure, as figure 1 , where a and b are the displacements of different hydrogens in the polymer, respectively.
[0063] The branched polylactic acid ob...
Embodiment 2
[0065] A 500mL reaction bottle with two vents was vacuum-baked and degassed, and after repeated argon filling three times, 200g (1.4mol) lactide, 0.26g (2.8×10 -3 mol) glycerol with 0.28g (7×10 -4 mol) stannous octoate, sealed system, heated to 120°C, stirred and reacted for 24 hours to obtain a white crystalline polymer, dissolved in chloroform, precipitated with excess ethanol to obtain a white solid, dried in vacuum to constant weight, and obtained branched polylactic acid , the relative weight average molecular weight is 92.0kg / mol, the relative molecular weight distribution is 1.48, and the relative molecular weight distribution is a relatively narrow peak.
[0066] The branched polylactic acid obtained in Example 2 was analyzed by nuclear magnetic resonance, and the number of branched chains was 3 through analytical calculation.
[0067] The branched polylactic acid obtained in Example 2 was analyzed by differential scanning calorimetry (DSC), and its melting point was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com