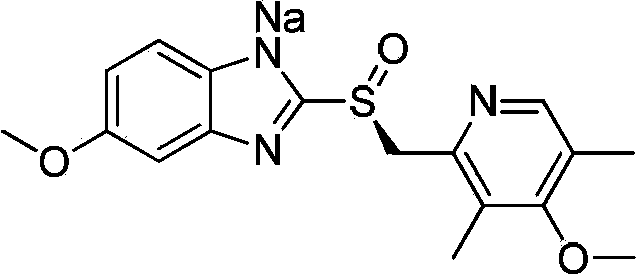
Preparation method of esomeprazole sodium
A technology of esomeprazole sodium and omeprazole, which is applied in the preparation field of preparation methods, can solve the problems of unsuitability for large-scale industrial production, dark color of inclusion complexes, and great toxicity to human body, and achieve appearance quality Good, good quality, less environmental pollution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
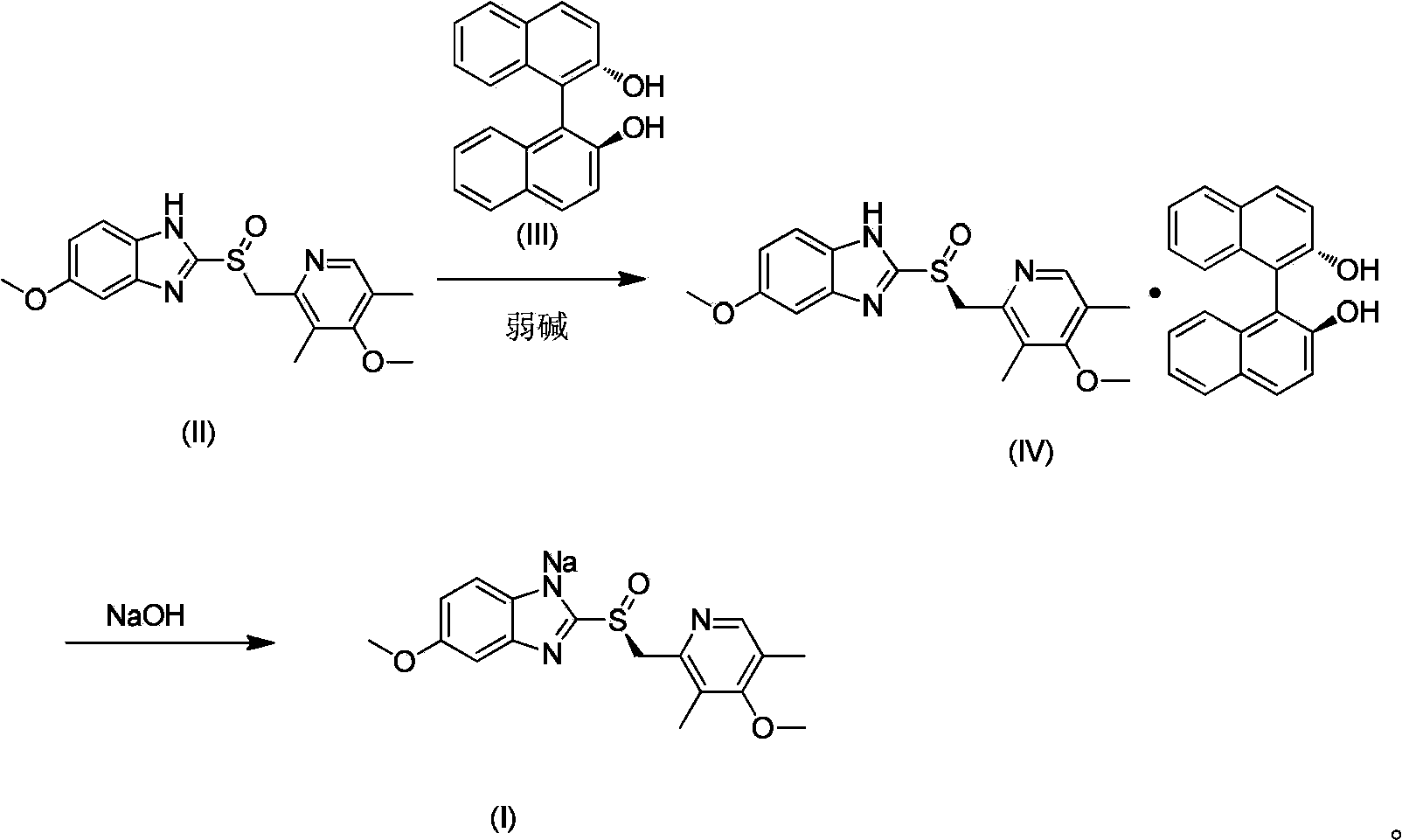
[0037] Example 1 Preparation of esomeprazole and (S)-(-)-[1,1'-binaphthyl]-2,2'-diphenol inclusion complex (IV).
[0038] In a 20L reactor, add racemic omeprazole 3.0kg (8.685mol), (S)-(-)-[1,1'-binaphthyl]-2,2'-diphenol 1.492kg (5.211mol), triethylamine 0.879kg (8.685mol) and absolute ethanol 12L, heat to 75°C and heat-preserve to dissolve, after dissolving, heat-preserve and stir at 60°C for 2h; cool to 30°C to crystallize, filter the suspended solid, filter cake After washing with 6L of ethanol and vacuum drying at 50°C, 2.442kg of off-white solid was obtained. Yield: 89%, HPLC purity (normalized method): 98.69%, optical purity (e.e%): 99.43%.
Embodiment 2
[0039] Example 2 Preparation of esomeprazole and (S)-(-)-[1,1'-binaphthyl]-2,2'-diphenol inclusion complex (IV).
[0040] In a 50L reactor, add racemic omeprazole 5.0kg (14.475mol), (S)-(-)-[1,1'-binaphthyl]-2,2'-diphenol 2.488kg (8.685mol), triethylamine 1.465kg (14.475mol) and 2-propanol 25L, heat to 70°C and heat-preserve to dissolve, after dissolving, keep stirring at 55°C for 3 hours; cool to 35°C for crystallization, filter the suspended solid, and filter The cake was washed with 10 L of 2-propanol and dried under vacuum at 50°C to obtain 4.001 kg of off-white solid. Yield: 87.5%, HPLC purity (normalized method): 98.34%, optical purity (e.e.%): 99.55%.
Embodiment 3
[0042] In a 50L reactor, add racemic omeprazole 5.0kg (14.475mol), (S)-(-)-[1,1'-binaphthyl]-2,2'-diphenol 2.07kg (7.2375mol), 4.3425mol of diethylamine and 15L of methanol, heated to 65°C and kept to dissolve, then stirred at 50°C for 2h after dissolving; cooled to 40°C for crystallization, filtered the suspended solid, washed the filter cake with 10L of methanol and placed in After vacuum drying at 50°C, 4.0 kg of off-white solid was obtained. Yield: 87.9%, HPLC purity (normalized method): 98.36%, optical purity (e.e%): 99.32%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com