Method for preparing L-tertiary leucine
A technology of tert-leucine and leucine dehydrogenase, which is applied in the field of high-efficiency preparation of L-tert-leucine with single optical purity, can solve the problems of serious environmental pollution, harsh reaction conditions, and complicated reaction process, and achieve high Effects of economic value, low cost, and cost reduction of coenzymes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
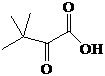
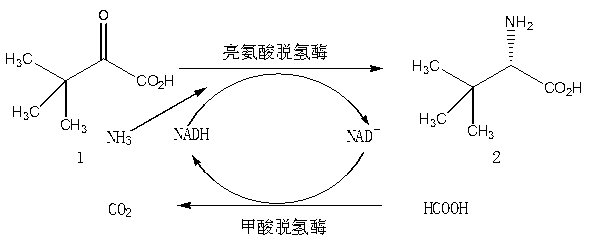
[0037] In a 10 mL reaction system, the substrate trimethylpyruvate, ammonium formate, ammonium formate-ammonia buffer, NAD + , leucine dehydrogenase, formate dehydrogenase, the reaction system contains: substrate trimethylpyruvate 1.0M (130g / L), ammonium formate 1.0M (63g / L), 0.1 M (pH 8.0) Ammonium formate-ammonia buffer, NAD + 0.01mM (0.007 g / L), leucine dehydrogenase 2.0 U / mL, formate dehydrogenase 2.0 U / mL, maintain the pH with ammonia water and hydrochloric acid, stir the reaction at room temperature (25°C) for 3 hours, after the reaction, Heat to 80°C and maintain for 30 minutes to fully denature the protein in the reaction solution, centrifuge for 15 min to remove the denatured protein, remove the solvent, and filter to obtain the L-tert-leucine product.
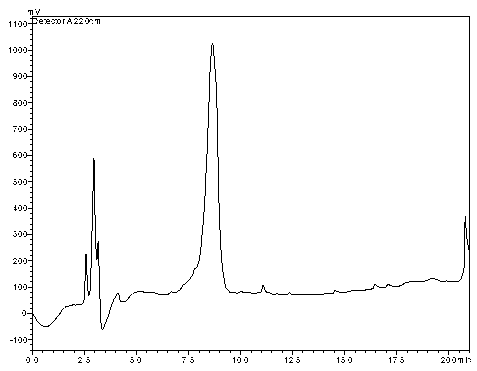
[0038] The substrate conversion was analyzed by high performance liquid chromatography. The results showed that the substrate was completely converted to the product L-tert-leucine.
[0039] The conversion rate an...
Embodiment 2
[0041] Prepare a 10 mL reaction system, which includes: substrate trimethylpyruvate 2.0M (260 g / L), ammonium formate 3.0M (189g / L), 0.1 M (pH 8.5) ammonium chloride-ammonia water buffer, NAD + 0.04mM (0.03 g / L), leucine dehydrogenase 3.0 U / mL, formate dehydrogenase 4.6 U / mL, shake at room temperature for 24 hours. Other operations were performed as in Example 1 to obtain the white product L-tert-leucine, and the conversion of the substrate was complete.
Embodiment 3
[0043] Prepare a 100 mL reaction system, which includes: substrate trimethylpyruvate 1.5M (195 g / L), ammonium formate 3.0M (189g / L), 0.1 M (pH 8.5) Tris-HCl buffer , NAD + 0.1mM (0.07 g / L), leucine dehydrogenase 3.0 U / mL, formate dehydrogenase 4.6 U / mL, shake at room temperature for 24 hours. Other operations were performed as in Example 1 to obtain the white product L-tert-leucine, and the conversion of the substrate was complete.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com