Celecoxib chewable tablet and preparation method thereof
A technology for celecoxib and chewable tablets, applied in the field of celecoxib chewable tablets and preparation thereof, can solve the problems of poor bioavailability, small degree of disintegration, inconvenience in taking and the like, and achieves smooth and beautiful tablet surface , The effect of promoting fluidity and large adsorption force
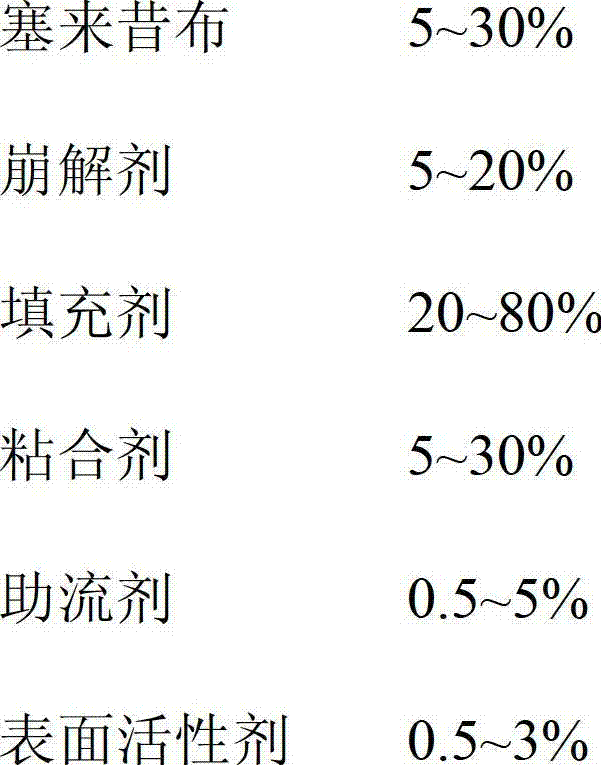
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020]
[0021] Preparation:
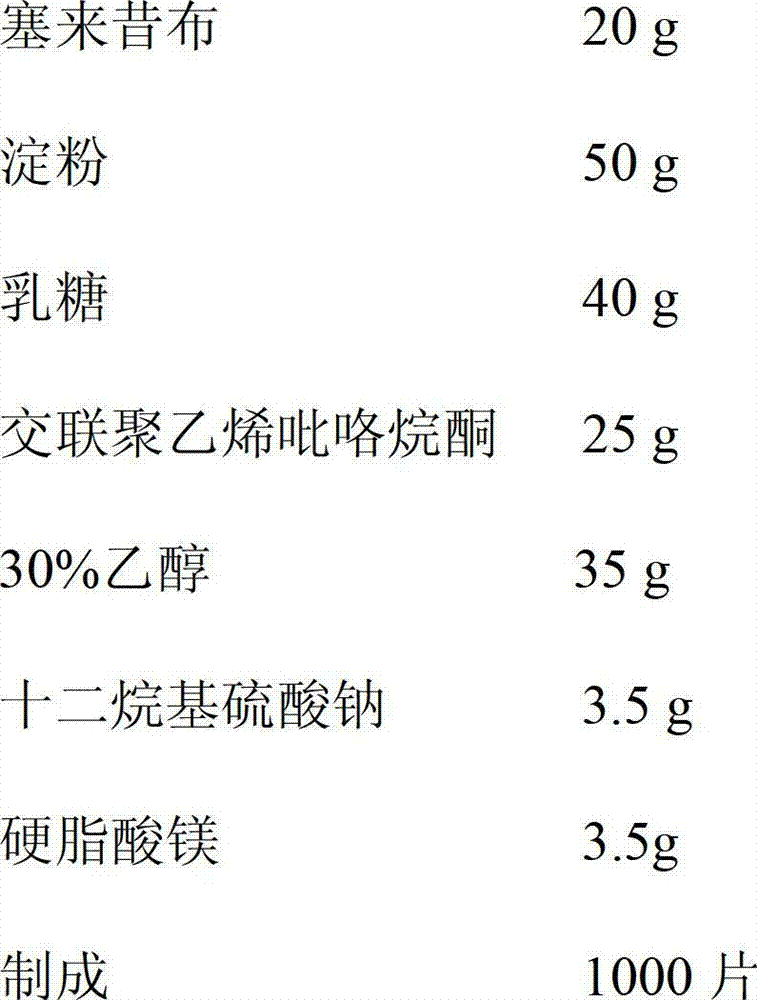
[0022] Pass celecoxib through a 100-mesh sieve, starch, lactose, and cross-linked polyvinylpyrrolidone through a 80-mesh sieve, and set aside; accurately weigh 20 g of celecoxib, 50 g of starch, 40 g of lactose, 25 g of cross-linked polyvinylpyrrolidone, and Mix 3.5g of sodium alkyl sulfate evenly; then add 35g of ethanol with a volume concentration of 30% to make a soft material, pass through a 18-20 mesh sieve to make granules, and dry the granules in an oven at 55°C; pass the dry granules through a 20-mesh sieve for granulation, Add 3.5 g of magnesium stearate and mix well. After inspection of the semi-finished product, it is pressed into tablets. After the finished product is inspected, it is packaged and ready to be obtained.
[0023] The celecoxib chewable tablets prepared in this example were inspected, and its content, properties, identification, disintegration time limit, dissolution rate, dispersion uniformity and other quality ind...
Embodiment 2
[0025]
[0026] Preparation:
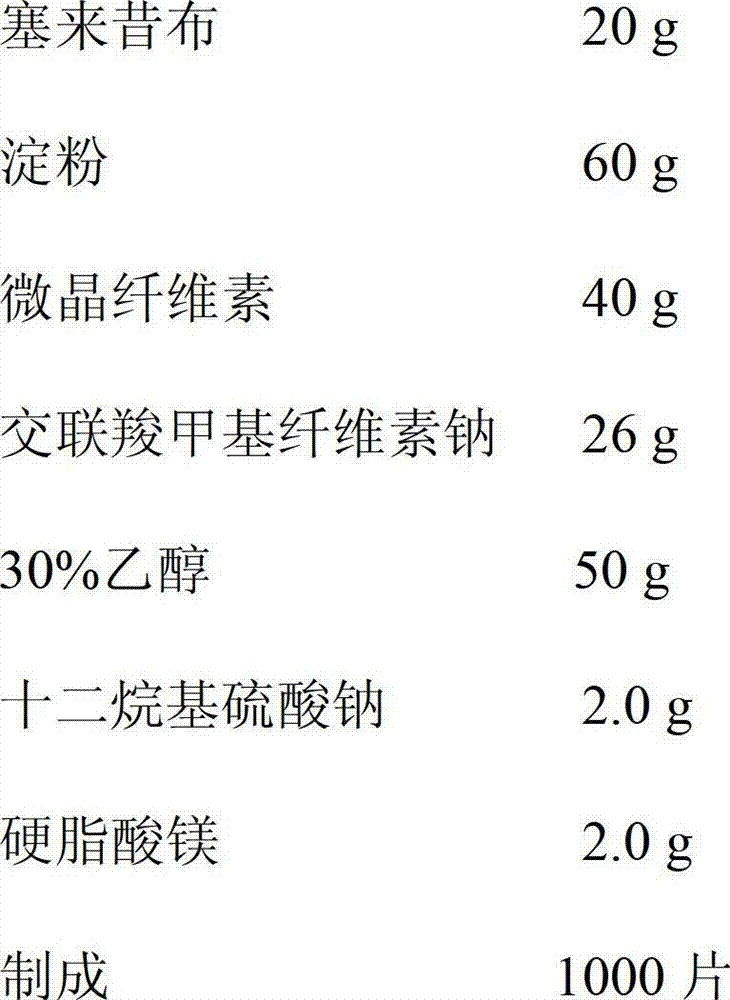
[0027] Pass celecoxib through a 100-mesh sieve, starch, cellulose powder, and sodium carboxymethyl cellulose through a 80-mesh sieve, and set aside; accurately weigh 20 g of celecoxib, 50 g of microcrystalline cellulose, and croscarmellose Mix 40g of plain sodium evenly; then add 50g of ethanol with a volume concentration of 30% to make a soft material, pass through a 18-20 mesh sieve to make granules, and dry the granules in an oven at 65°C; pass the dry granules through a 20 mesh sieve for granulation, add stearin Magnesium acid 2.0g and mix well. After inspection of the semi-finished product, it is pressed into tablets. After the finished product is inspected, it is packaged and ready to be obtained.
[0028] The celecoxib chewable tablets prepared in this example, after inspection, its content, character, identification, disintegration time limit, dissolution rate, dispersion uniformity and other quality indicators not only conform to th...
Embodiment 3
[0030]
[0031] Preparation:
[0032] Pass celecoxib through a 100-mesh sieve, starch, microcrystalline cellulose, and croscarmellose sodium through a 80-mesh sieve, and set aside; accurately weigh 20 g of celecoxib, 30 g of microcrystalline cellulose, and cross-linked carboxymethyl cellulose. 40g of sodium methylcellulose, 25.0g of low-substituted hydroxypropyl cellulose, mix evenly; then add 44g of ethanol with a volume concentration of 30% to make a soft material, pass through a 18-20 mesh sieve to make granules, and granules are placed in an oven at 70°C Drying; the dry granules are passed through a 20-mesh sieve for granulation, and 2.0 g of magnesium stearate is added and mixed evenly. After inspection of the semi-finished product, it is pressed into tablets. After the finished product is inspected, it is packaged and ready to be obtained.
[0033] The celecoxib chewable tablets prepared in this example were inspected, and its content, properties, identification, disi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com