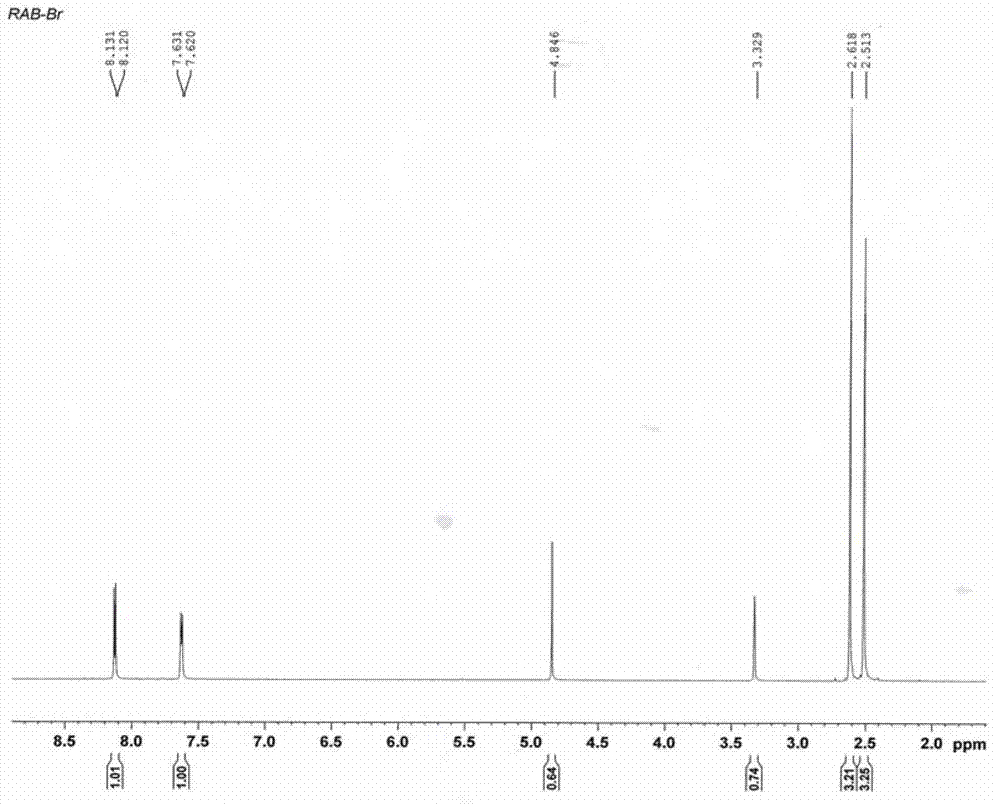
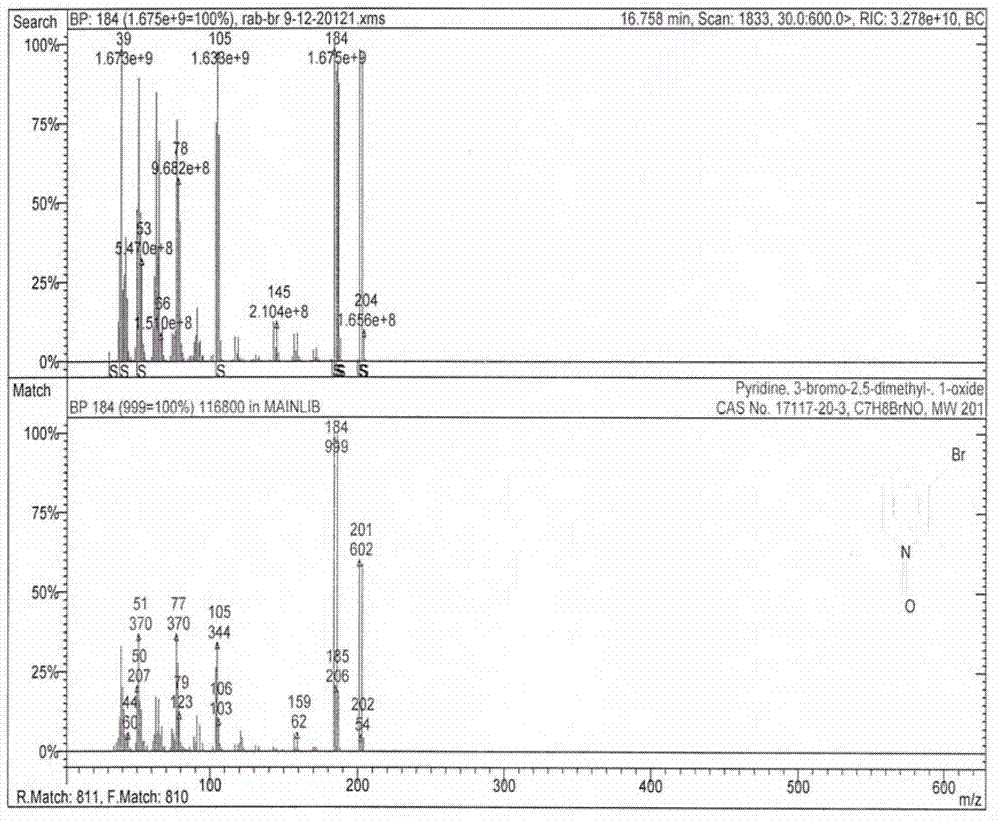
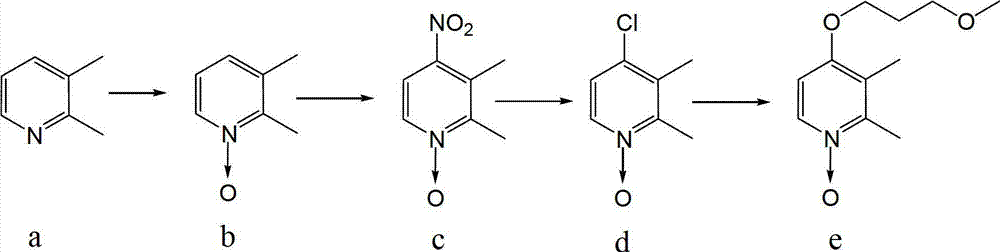
Method for preparing 4-(3-methoxy propoxy)-2,3-dimethyl pyridine-N-oxide
A technology of methoxypropoxy and lutidine, applied in the field of organic chemical synthesis, can solve the problems of shortening reaction steps, many side reactions unfavorable to industrialization, long reaction time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The invention provides a preparation method of 4-(3-methoxypropoxy)-2,3-dimethylpyridine-N-oxide, comprising the following steps: A) 2,3-dimethyl Pyridine-N-oxide, bromine source and solvent are mixed, heated and reacted to obtain 2,3-dimethyl-4-bromopyridine-N-oxide; B) the 2,3-dimethyl-4- Bromopyridine-N-oxide is mixed with 3-methoxypropanol and reacted with heating to obtain 4-(3-methoxypropoxy)-2,3-dimethylpyridine-N-oxide.
[0033] The 2,3-lutidine-N-oxide can be purchased from the market or self-made, and there is no special limitation. The 2,3-lutidine-N-oxide described in the present invention is preferably prepared according to the following steps: 2,3-lutidine, glacial acetic acid and hydrogen peroxide are mixed, heated and reacted to obtain 2,3-di Pyridine-N-oxide. The molar ratio of the 2,3-lutidine to hydrogen peroxide is 1:1.5~3, preferably 1:2~2.5, and the molar ratio of the 2,3-lutidine to glacial acetic acid is 1:1.5 ~2.5, preferably 1:1.8~2.2.
[0...
Embodiment 1
[0059] 1.1 Add 60g of glacial acetic acid to a 500ml four-neck flask, control the temperature not higher than 50°C, add 53.5g (0.5mol) 2,3-lutidine, stir for 30min, slowly raise the temperature to 80°C, add 85g within 2h (1.25mol) hydrogen peroxide (50%), then heated up to 93°C, reacted for 5h, distilled off water and unreacted glacial acetic acid under reduced pressure (the temperature should not exceed 100°C), and obtained wine red liquid 2,3-lutidine -N-oxide 60.3g, calculated yield is 98%.
[0060] 1.2 Mix 60.3g (0.49mol) of 2,3-lutidine-N-oxide obtained in 1.1, 112g (0.81mol) of potassium carbonate and 500ml of carbon tetrachloride, raise the temperature to reflux and drop slowly within 5h Add 116g (0.73mol) of bromine, continue to reflux for 2 hours after addition, and cool down to room temperature, add a small amount of 10% sodium bisulfite solution until the dyeing of the reaction solution becomes lighter, filter, recover potassium bromide, and distill the filtrate to ...
Embodiment 2
[0070] 2.1 Add 60g of glacial acetic acid to a 500ml four-necked flask, control the temperature not higher than 50°C, add 53.5g (0.5mol) 2,3-lutidine, stir for 30min, slowly raise the temperature to 80°C, add 68g within 2h (1.0mol) hydrogen peroxide (50%), then heat up to 93°C, react for 5h, cool down to 80°C, add 17g (0.25mol) hydrogen peroxide (50%) dropwise, heat up to 93°C, continue to react for 3h, and remove by distillation under reduced pressure Water and unreacted glacial acetic acid (the temperature must not exceed 100°C) gave 60.3 g of wine-red liquid 2,3-lutidine-N-oxide, and the calculated yield was 98%.
[0071] 2.2 Mix 60.3g (0.49mol) of 2,3-lutidine-N-oxide obtained in 2.1, 130g (0.94mol) of potassium carbonate and 500ml of dichloroethane, raise the temperature to reflux and drop slowly within 5h Add 150g (0.94mol) of bromine, continue to reflux for 8 hours after adding, cool down to room temperature, add a small amount of 10% sodium bisulfite solution until the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com