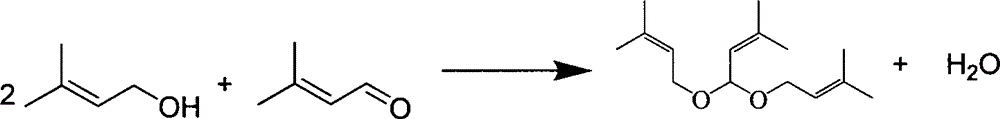Unsaturated acetal preparation method
An unsaturated and acetal technology, applied in the field of preparation of high boiling point unsaturated acetal, can solve the problems of increased side reactions, strong corrosion of lithium chloride and high cost, and achieves increased reaction rate, stable process state and equipment requirements. low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
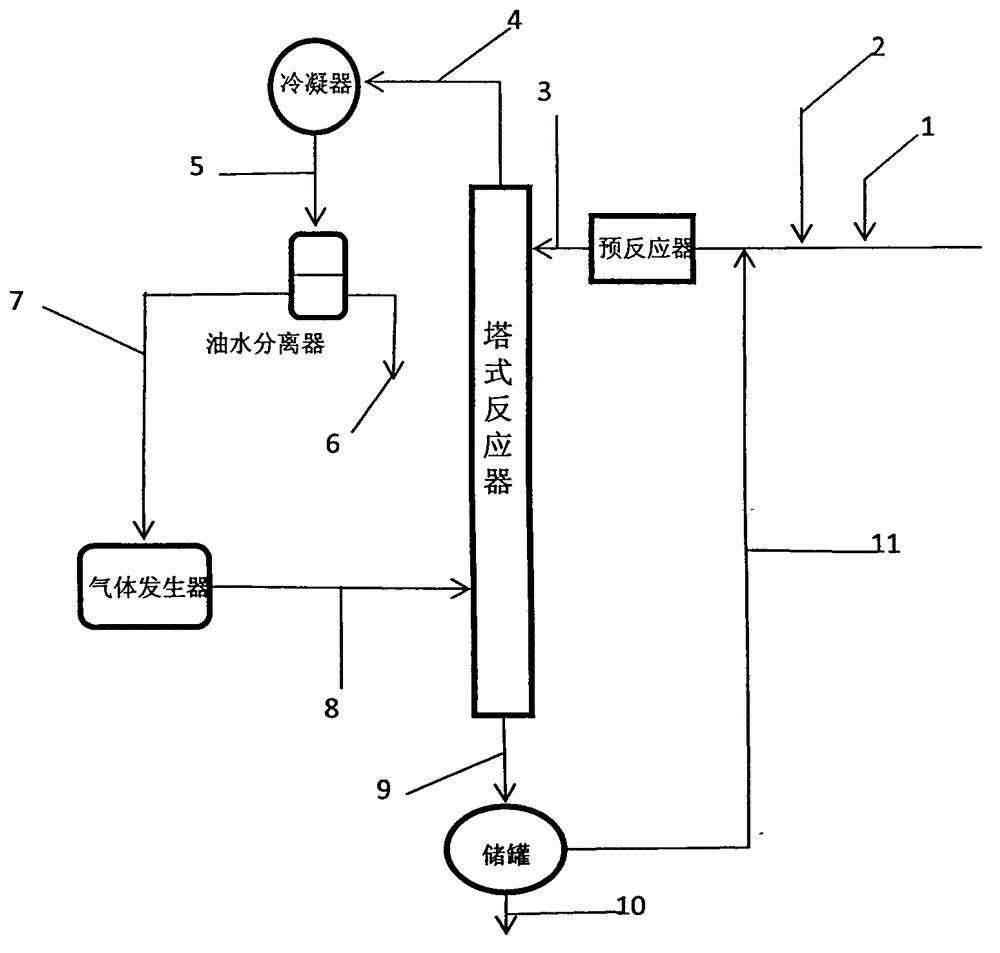
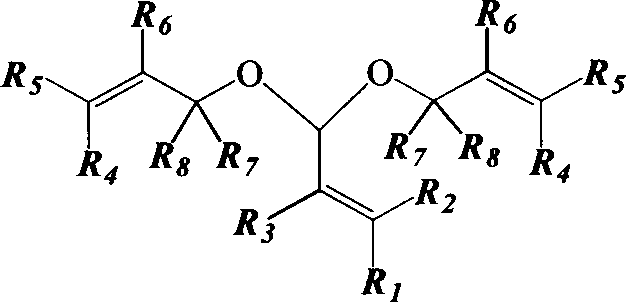
[0061] The tower reactor is a rectification tower with an inner diameter of 25mm, a 0.5m Raschig ring filler is installed on the top, and a 0.5m carboxylic acid type acidic ion exchange resin (ion exchange resin produced by Liaoning Dandong Mingzhu Special Resin Co., Ltd.) is installed in the middle. D113), the lower end is filled with Raschig ring packing with a height of 0.5m, and the number of effective trays is 23. A condenser and an oil-water separator are installed on the top of the rectification column. First, cyclohexane is pumped into the gas generator to prepare cyclohexane vapor at 85°C and 115KPa, which is passed into the rectification tower to preheat the reaction system. Prenol and prenyl aldehyde are dosed according to a mass ratio of 2.5:1 (molar ratio is 2.44:1), and mixed uniformly to obtain a reaction liquid. When the temperature at the top of the tower is greater than 82°C, add the reaction liquid to the fixed-bed reactor equipped with carboxylic acid-type...
Embodiment 2
[0063] The above-mentioned carboxylic acid type acidic ion exchange resin filler is changed into heteropolyacid catalyst and repeats above-mentioned experiment, and the content of acetal is 74.62wt% in the extraction liquid of tower still, and prenaldehyde content is 2.08wt%, prenol Content is 20.42wt%, and the content of cyclohexane is 0.84wt%, and other impurity content is 2.04wt%, and this illustrates that the conversion ratio of prenaldehyde is 93.15%, and 3-methyl-2-butene-1-aldehyde The selectivity to di-isopentenyl acetal was 93.04%.
Embodiment 3
[0065] The reaction device is a rectifying tower with an internal diameter of 25mm, a 0.3m Raschig ring filler is installed on the top, and a 1m carboxylic acid type acidic ion-exchange resin (ion-exchange resin D113 produced by Liaoning Dandong Mingzhu Special Resin Co., Ltd.) is installed in the middle. The lower end is filled with Raschig ring packing with a height of 0.2m, and the number of effective trays is 12. The top of the tower is equipped with a condenser and an oil-water separator. First, pump 1,2-dichloroethane into the gas generator to prepare 1,2-dichloroethane vapor at 85°C and 105.19KPa, and pass it into the rectification tower to preheat the reaction system . 2-Methyl-3-buten-2-ol and prenal are dosed according to a mass ratio of 2.5:1 (molar ratio is 2.44:1), and mixed evenly. When the temperature at the top of the tower is higher than 80°C, add the reaction liquid to the fixed-bed reactor equipped with carboxylic acid type acidic ion exchange resin, prehe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com