Sinomenine derivate and preparation method and application thereof
A technology of sinomenine and its derivatives, which is applied in the field of drug synthesis, can solve rare problems, and achieve the effects of low cytotoxicity, good transdermal performance, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
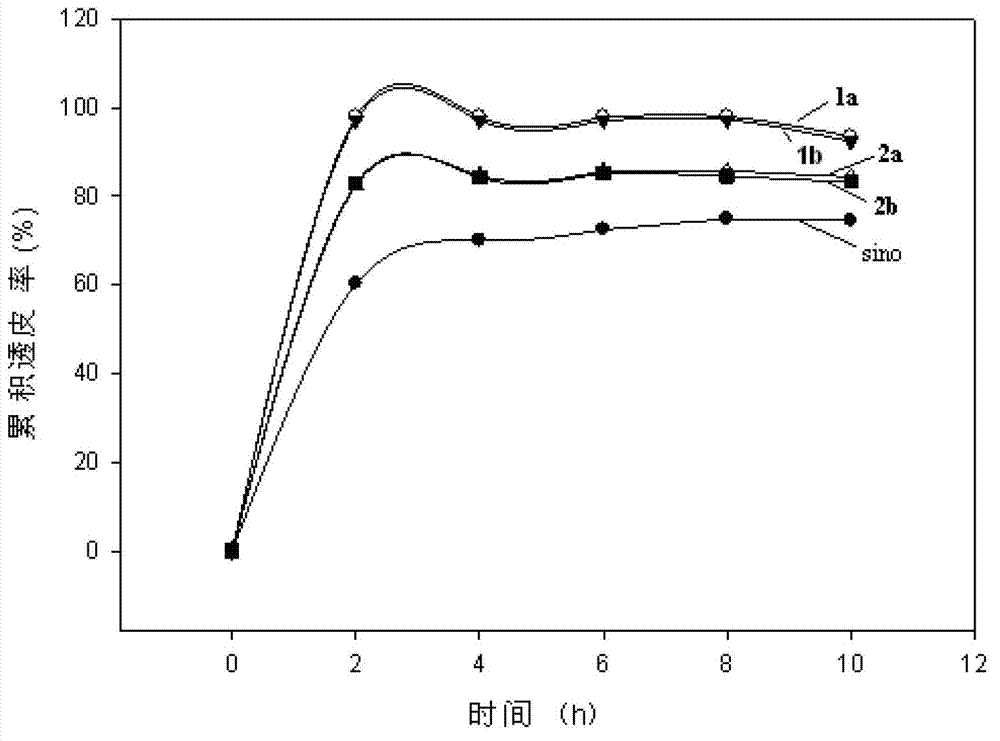
[0039] Embodiment 1: the synthesis of compound 1a
[0040] Using pyridine as solvent, 0.246g (0.684mmol) sinomenine benzyl alcohol intermediate and 1mL acetic anhydride were refluxed for 3h under the catalysis of DMAP; pyridine was evaporated under reduced pressure, and saturated sodium bicarbonate solution was added to adjust the pH value to 8~9 Finally, extract with chloroform; the organic phase is washed with water several times and then dried with anhydrous sodium sulfate. The organic phase is evaporated and spin-dried under reduced pressure, and the residue is separated and purified by column chromatography [V (dichloromethane): V (methanol )=30:1] to obtain compound 1a. Yield 85.2%.
[0041] Compound 1a: pale yellow solid, mp.56~58°C; 1 H NMR (400MHz, CDCl 3 )δ=1.86~1.93(m,1H),2.11(s,3H),2.04~2.16(m,2H),2.36(s,3H),2.43(s,3H),2.51~2.55(m,2H) ,2.66(dd,J 1 =6.0Hz&J 2 =18.8Hz,1H),3.02(t,J=7.2Hz,2H),3.27(t,J=4.0Hz,1H),3.48(s,3H),3.76(s,3H),3.89(d,J =16.0Hz,1H),5.07(s,2...
Embodiment 2
[0042] Embodiment 2: the synthesis of compound 1b:
[0043] The procedure is the same as in Example 1, 0.248g, (0.684mmol) sinomenine benzyl alcohol intermediate and 1.5mL propionic anhydride were used as raw materials to prepare compound 1b. Yield 75.2%.
[0044] Compound 1b: pale yellow solid, mp.58~60°C; 1 H NMR (400MHz, CDCl 3 ):δ=1.29(t,J=7.6Hz,3H),1.59~1.62(m,1H),1.71(t,J=7.6Hz,3H),1.83~1.94(m,2H),2.04~2.09( m,1H),2.38(q,J=7.6Hz,2H),2.42(s,3H),2.49~2.54(m,2H),2.57~2.73(m,3H),2.99~3.03(m,2H) ,3.26(t,J=4.0Hz,1H),3.48(s,3H),3.74(s,3H),3.89(d,J=16.0Hz,1H),5.08(d,J=2.0Hz,2H) ,5.46(d,J=1.6Hz,1H),6.85(s,1H). 13 C NMR (100MHz, CDCl 3 ):δ=9.14,20.85,27.59,27.89,36.92,40.74,42.68,45.61,46.63,50.09,54.80,55.94,56.01,64.02,112.34,114.76,128.69,130.31,130.53,139.72,149.63,152.50,174.05, 192.19.MS(EI)m / z(%):471.3(M + ,93).
Embodiment 3
[0045] Embodiment 3: the synthesis of compound 2a
[0046] With DMF as solvent, in Cs 2 o 3 Under catalysis, 0.347g (0.966mmol) of sinomenine benzyl alcohol intermediate and 0.27ml (2.90mmol) of 1-bromopropane were stirred and reacted at 60°C for 2h; after filtration, the solvent was evaporated under reduced pressure, and the residue was subjected to column chromatography Compound 2a was obtained by separation and purification [V (dichloromethane): V (methanol) = 15: 1]. Yield 80.6%.
[0047] Compound 2a: colorless oil; 1 H NMR (400MHz, CDCl 3 ):δ=1.04(t,J=7.6Hz,3H),1.83~1.90(m,2H),1.95~1.98(m,1H),2.03~2.15(m,3H),2.42(bs,1H), 2.50(s,3H),2.53(d,J=16.0Hz,2H),2.72~2.80(m,2H),3.07~3.11(m,1H),3.21(s,1H),3.41(t,J= 4.0Hz,1H),3.48(s,3H),3.80(s,3H),3.97~4.10(m,2H),4.21(d,J=16.0Hz,1H),4.61(q,J=10.8Hz, 2H),5.45(d,J=1.6Hz,1H),6.87(s,1H). 13 C NMR (100MHz, CDCl 3 ):δ=10.44,20.96,23.53,36.79,40.81,42.57,45.41,47.20,49.69,54.84,55.79,56.34,63.43,73.80,111.69,114.90,130.18,132.053,14...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com