Preparation method of compound dienogest
A technology for dienogest and compound, which is applied in the field of compound preparation, can solve the problems of high price, low yield, unsuitability for industrialized large-scale production and the like, and achieves the effects of cheap raw materials and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
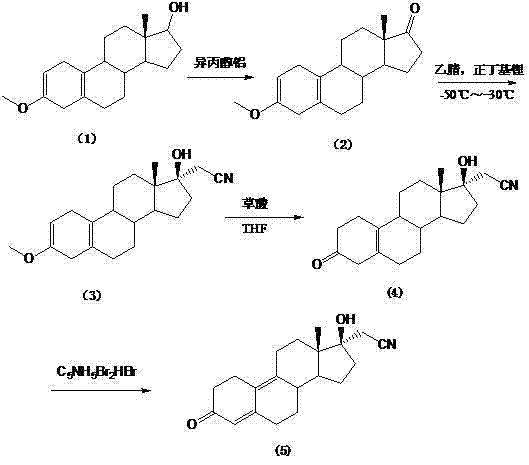
[0041] The preparation method of compound dienogest of the present invention comprises the following steps:
[0042] The first step is to prepare 17α-cyanomethyl-17β-hydroxyl-5(10), 9(11)-estradiene-3,17-dione-3,3-ethylene ketal (formula 2 );
[0043] Put tetrahydrofuran into the reaction bottle, lower the temperature to below -40°C, add n-butyllithium solution, dropwise add the mixed solution of acetonitrile and tetrahydrofuran, stir for 15 minutes, then add the raw materials estra-5(10), 9(11 )-diene-3,17-dione-3,3-ethylene ketal (Formula 1) and tetrahydrofuran mixed solution, the temperature is controlled between -80 ~ -20 ° C, preferably -50 ~ -30 ℃, stirred for 0.5 hours, after the reaction was complete, water was added dropwise to terminate the reaction, the organic layer was separated, washed with water, dehydrated and concentrated to obtain compound formula (2), with a purity of more than 96%;
[0044] The second step is to prepare 17α-cyanomethyl-17β-hydroxyl-13β-me...
Embodiment 1
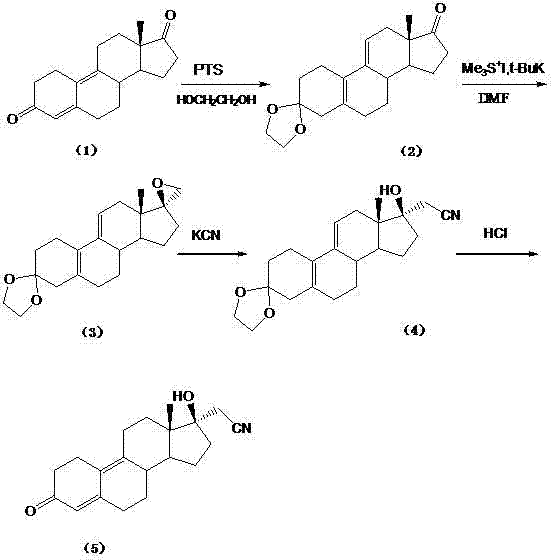
[0053] Example 1: Preparation of 17α-cyanomethyl-17β-hydroxyl-5(10), 9(11)-estradiene-3,17-dione-3,3-ethylene ketal (Formula 2 )
[0054] Add 450ml of tetrahydrofuran into a 3L four-neck flask, cool down to below -50°C, slowly add 525ml of n-butyllithium solution, the reaction is exothermic, stir to adjust the temperature between -80~-20°C, add dropwise 300ml of tetrahydrofuran and 130ml of acetonitrile mixed solution, after dropping, stir for 15 minutes; dissolve 148g of estro-5(10), 9(11)-diene-3,17-dione-3,3-ethylene with 900ml of dry tetrahydrofuran Base ketal (Formula 1), dropwise into the reaction bottle between -80~-20°C, after dropping, stir for 0.5 hours, adjust the temperature below -40°C, add 400ml of water dropwise, stir for 1 hour, stand still Separate the layers and collect the organic phase. The organic phase was washed twice with 800 ml of water, dehydrated, and concentrated to dryness under reduced pressure at 40°C to obtain an oily substance (compound formul...
Embodiment 2
[0055] Example 2: Preparation of crude 17α-cyanomethyl-17β-hydroxyl-13β-methylstan-4,9-dien-3-one (Formula 3)
[0056] With 17α-cyanomethyl-17β-hydroxyl-5(10), 9(11)-estradiene-3,17-dione-3,3-ethylene ketal (formula 2) according to Add 200ml of methanol to a 1000ml reaction bottle of 48g of formula (1), stir to dissolve, add 10ml of hydrochloric acid at room temperature, stir for 1.5 hours, concentrate to a small volume at 40°C, water analysis, filter, and wash with water to obtain 48g of a yellow solid , which is the crude product of dienogest with a purity (HPLC) of 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com