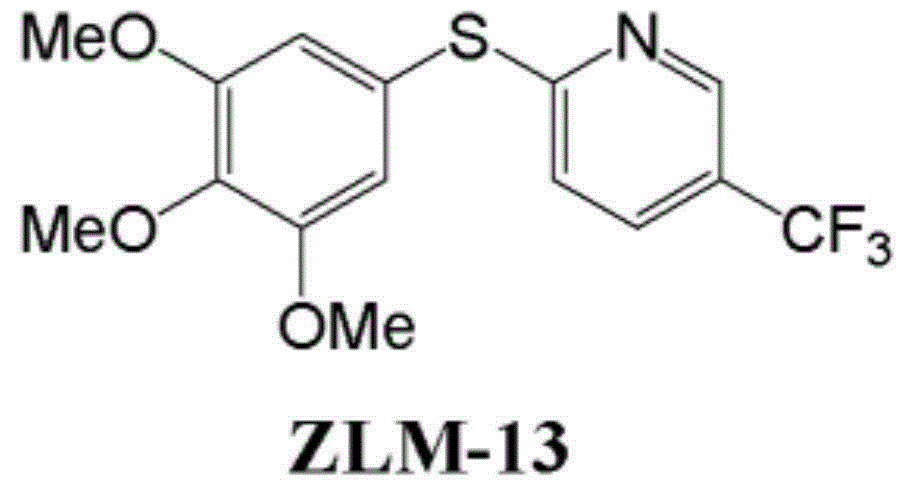
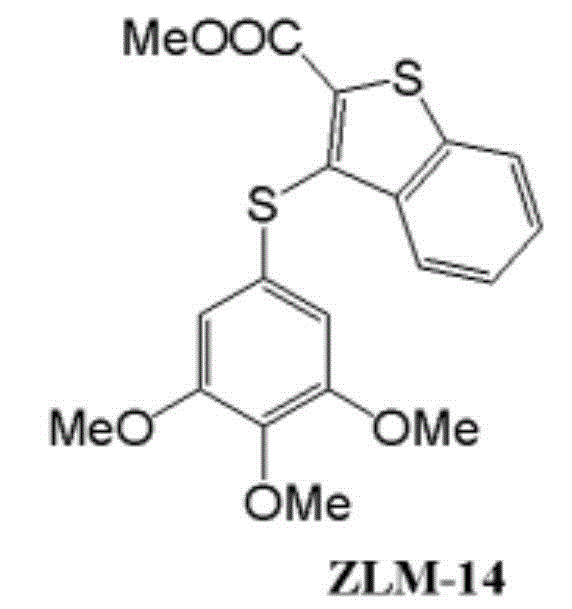
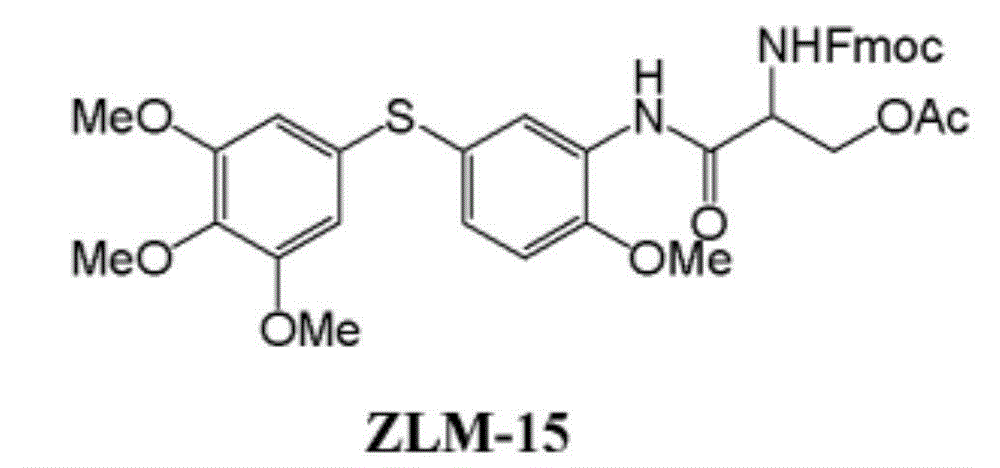
Method for manufacturing polymer used as electron transfer layer of solar cell
An electron transport layer, solar cell technology, applied in the field of polymer manufacturing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Preparation of O-ethyl-S-(3,4,5-trimethoxyphenyl)dithiocarbonate (ZLM-1)
[0026]
[0027] Dissolve 3,4,5-trimethoxyaniline (22.3g) in 30.5mL of concentrated hydrochloric acid and 250mL of water, cool to about 0°C in an ice bath, slowly add NaNO dropwise under stirring 2 Aqueous solution (9.2g), temperature controlled not to exceed 5°C, continued reaction for 15min after dropping, then added AcONa (20.0g). This diazonium salt solution was slowly added dropwise to KS2COEt aqueous solution (48.7g dissolved in 120mL water) at about 80°C, and reacted at 70-80°C for 1h. After the reaction was completed, cool to room temperature, extract 100 mL×3 with EA, and combine the organic phases. Then dry with anhydrous sodium sulfate. After suction filtration, the filtrate was obtained, and the solvent was spun off to obtain 30.4 g of a reddish-brown oil, which was separated on a silica gel column to obtain 11.8 g of light yellow-white viscous solid, with a yield of 34%. 1 H NMR...
Embodiment 2
[0029] Preparation of 3,4,5-trimethoxy-1-[(3-amino-4-nitrophenyl)thio]benzene (ZLM-2)
[0030]
[0031] Dissolve O-ethyl-S-(3,4,5-trimethoxyphenyl)dithiocarbonate ZLM-1 (4.0g) in THF (66mL), slowly add LiAlH in portions 4 (2.11g), refluxed for 1h, cooled to room temperature, adjusted to pH=5 with 10% HCl, then extracted 100mL×3 with EA, combined the organic phases, dried with anhydrous sodium sulfate for 1h, and then filtered with suction to obtain the filtrate, which was spun The solvent is removed to obtain 3,4,5-trimethoxythiophenol. Under nitrogen protection, K 2 CO 3 (5.8g) and 5-chloro-2-nitroaniline (1.2g) were added to a 50mL two-necked flask, and then the 3,4,5-trimethoxythiophenol obtained in the previous step was dissolved in DMF (23.7mL) Add it into the reaction flask, reflux at 120°C, and monitor by TLC (developing solvent: PE:EA=4:1). The raw materials disappeared, the reaction was stopped, cooled to room temperature, diluted with 200 mL of water, extracte...
Embodiment 3
[0033] Preparation of 3,4,5-trimethoxy-1-[(3-acetamido-4-nitrophenyl)thio]benzene (ZLM-3)
[0034]
[0035]3,4,5-Trimethoxy-1-[(3-amino-4-nitrophenyl)thio]benzene ZLM-2 (50 mg) was added to a mixture of acetic anhydride (17 μL) and acetic acid (9 μL) In the solvent, reflux at 85-90°C for 2h. Low boiling point substances were distilled off under reduced pressure to obtain 61mg of yellow solid with a yield of 93%, mp 168.1-170.2°C. 1 H NMR (400MHz, CDCl 3 ): δ2.26(s, 3H), 3.87(s, 6H), 3.92(s, 3H), 6.73(dd, J=7.2, 1.8Hz, 1H), 6.81(s, 2H), 8.07(d, J=8.8Hz, 1H), 8.61(d, J=6.0Hz, 1H), 10.48(s, 1H). 13 C NMR (400MHz, CDCl3): δ25.7, 56.3, 61.0, 112.2, 117.6, 119.8, 123.7, 126.2, 132.9, 135.2, 139.6, 151.4, 154.0, 168.9. MS (EI) m / z: 378 (M + ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com