Pyridine-fluorene organic electrophosphorescence main body luminescent material and preparation method thereof
A phosphorescent host, luminescent material technology, applied in luminescent materials, organic chemistry, electrical solid devices, etc., can solve the problem of ineffective use of triplet exciton energy, achieve large steric hindrance effect, mild conditions, good photoelectric performance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
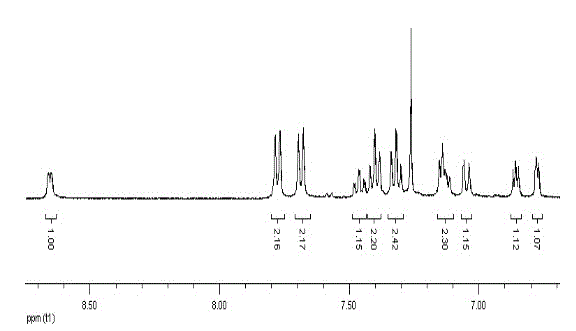
[0044] Example 1: 9-(2-pyridyl)-9-(2-thienyl)fluorene
[0045] 9-Hydroxy-9(2-pyridyl)fluorene (0.5180 g, 2 mmol), thiophene (1.28 mL, 16 mmol), and 200 mL of acetic acid were heated and stirred in turn to dissolve, then 4 mL of concentrated sulfuric acid was added and heated to 120°C for 24 h. After the reaction was cooled to room temperature, about 100 mL of cold water was added and stirred, then an aqueous sodium hydroxide solution was added until the solution was alkaline, extracted several times with dichloromethane, and the combined organic phases were dried over anhydrous magnesium sulfate. After drying, filter under reduced pressure and wash the desiccant with dichloromethane, and concentrate the obtained filtrate under reduced pressure with a rotary evaporator to remove most of the solvent to obtain a concentrated crude product. Then column chromatography, with ethyl acetate and petroleum ether as detergents, after purification, a white solid product was obtained (y...
Embodiment 2
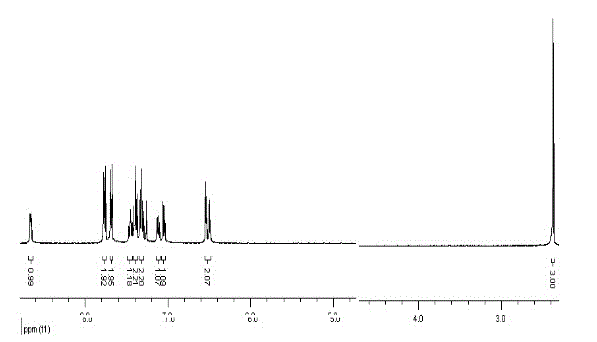
[0046] Embodiment 2: Synthesis of 9-(2-pyridyl)-9-(2-methylthienyl)fluorene:
[0047] 9-Hydroxy-9(2-pyridyl)fluorene (0.5180 g, 2 mmol), 2-methylthiophene (1.57 mL, 16 mmol), 200 mL of acetic acid were heated and stirred to dissolve in turn, and then 4 mL of concentrated sulfuric acid was added and heated to Reflux at 120°C for 24h. After the reaction was cooled to room temperature, about 100 mL of cold water was added and stirred, then an aqueous sodium hydroxide solution was added until the solution was alkaline, extracted several times with dichloromethane, and the combined organic phases were dried over anhydrous magnesium sulfate. After drying, filter under reduced pressure and wash the desiccant with dichloromethane, and concentrate the obtained filtrate under reduced pressure with a rotary evaporator to remove most of the solvent to obtain a concentrated crude product. Then column chromatography, with ethyl acetate and petroleum ether as detergents, after purificatio...
Embodiment 3
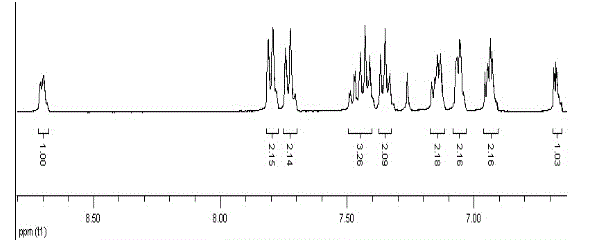
[0048] Example 3: Synthesis of 9-(2-pyridyl)-9-(2-(2,2-bithienyl))fluorene:
[0049] Take 9-hydroxy-9(2-pyridyl)fluorene (0.5180 g, 2 mmol), 2,2-dithiophene (0.1663 g, 1 mmol), 200 mL of acetic acid and heat and stir to dissolve in turn, then add 4 mL of concentrated sulfuric acid Heated to 120 ° C reflux for 24h. After the reaction was cooled to room temperature, about 100 mL of cold water was added and stirred, then an aqueous sodium hydroxide solution was added until the solution was alkaline, extracted several times with dichloromethane, and the combined organic phases were dried over anhydrous magnesium sulfate. After drying, filter under reduced pressure and wash the desiccant with dichloromethane, and concentrate the obtained filtrate under reduced pressure with a rotary evaporator to remove most of the solvent to obtain a concentrated crude product. Then column chromatography, with ethyl acetate and petroleum ether as detergents, after purification, a white solid pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com