Method for establishing prawn virus isolation, identification and infection model
A technology for virus isolation and model establishment, applied in the field of anti-virus research, it can solve problems that have not yet been reported, and achieve the effect of clear judgment criteria, obvious infection effect, and good reproducibility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] A method for isolation, identification and infection model establishment of shrimp virus, comprising the following steps:
[0021] (1) Obtainment of TSV virus tissue: The preserved TSV purified virus particles were diluted at a mass-to-volume ratio of 1:1000, and injected into the muscle of the third abdominal ganglion of Litopenaeus vannamei with an insulin syringe. ~12g), inject 10~12μL per tail (inject the virus at a dose of 1μL / g to each prawn), control the shrimp culture water temperature at 28-30℃, salinity 28‰ after injection, continuously aerate, observe the status of the prawns every day, Collect the dying prawns in time and place them in liquid nitrogen;
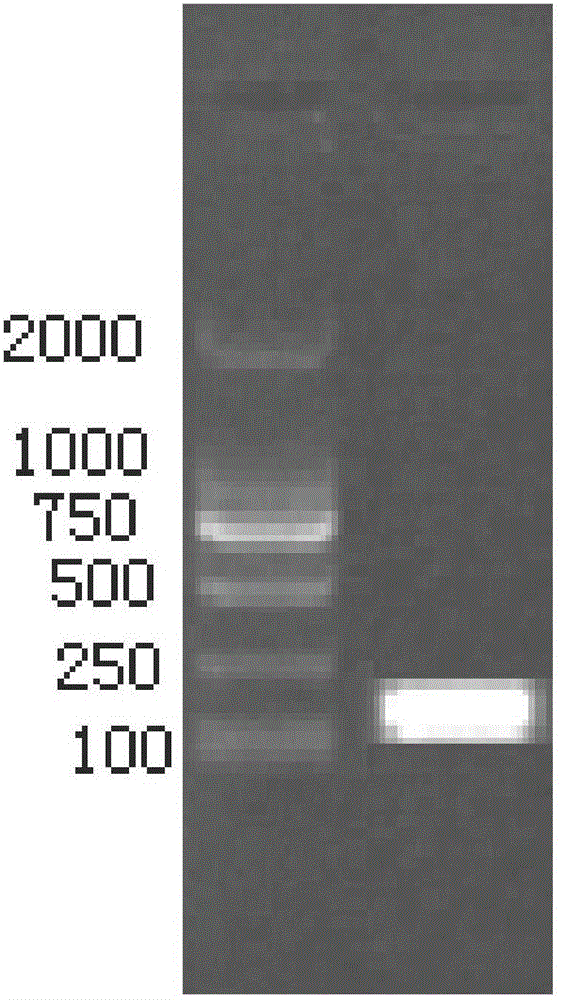
[0022] (2) TSV virus detection: PCR was used to detect TSV virus infection of shrimp, and RNA from hepatopancreatic tissue of dying shrimp was extracted, reverse transcribed into cDNA, and amplified using this cDNA as a template to amplify the upstream and downstream primer sequences of TSV virus. Upstream ...
Embodiment 2
[0028] A method for isolation, identification and infection model establishment of shrimp virus, comprising the following steps:
[0029] (1) Obtainment of TSV virus tissue: The preserved TSV purified virus particles were diluted at a mass-to-volume ratio of 1:1000, and injected into the muscle of the third abdominal ganglion of Litopenaeus vannamei with an insulin syringe. ~12g), inject 10~12μL per tail (inject the virus at a dose of 1μL / g to each prawn), control the shrimp culture water temperature at 28-30℃, salinity 28‰ after injection, continuously aerate, observe the status of the prawns every day, Collect the dying prawns in time and place them in liquid nitrogen;
[0030](2) TSV virus detection: PCR was used to detect TSV virus infection of shrimp, and RNA from hepatopancreatic tissue of dying shrimp was extracted, reverse transcribed into cDNA, and amplified using this cDNA as a template to amplify the upstream and downstream primer sequences of TSV virus. Upstream p...
Embodiment 3
[0036] A method for isolation, identification and infection model establishment of shrimp virus, comprising the following steps:
[0037] (1) Obtainment of TSV virus tissue: The preserved TSV purified virus particles were diluted at a mass-to-volume ratio of 1:1000, and injected into the muscle of the third abdominal ganglion of Litopenaeus vannamei with an insulin syringe. ~12g), inject 10~12μL per tail (inject the virus at a dose of 1μL / g to each prawn), control the shrimp culture water temperature at 28-30℃, salinity 28‰ after injection, continuously aerate, observe the status of the prawns every day, Collect the dying prawns in time and place them in liquid nitrogen;
[0038] (2) TSV virus detection: PCR was used to detect TSV virus infection of shrimp, and RNA from hepatopancreatic tissue of dying shrimp was extracted, reverse transcribed into cDNA, and amplified using this cDNA as a template to amplify the upstream and downstream primer sequences of TSV virus. Upstream ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com