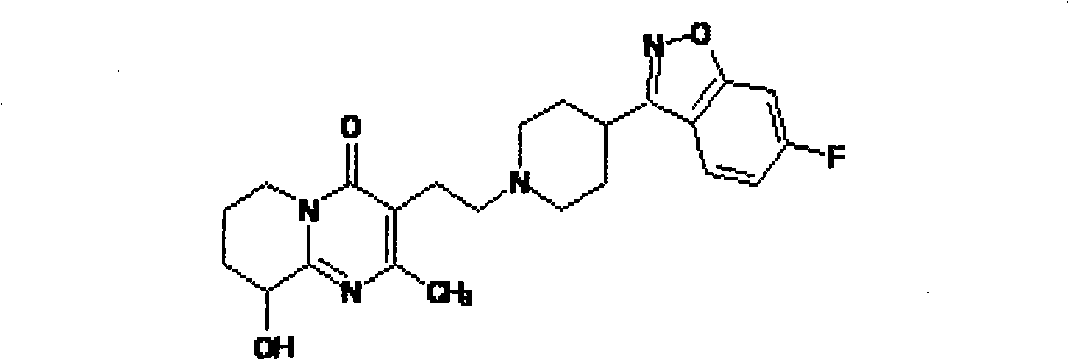
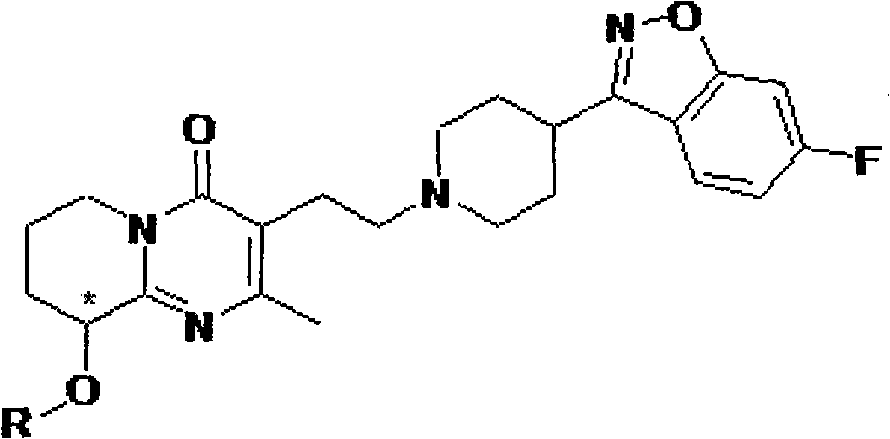
Paliperidone amino-acid ester and preparation method thereof
A technology of paliperidone amino acid ester and diperidone amino acid ester, which is applied in the field of paliperidone ester and its preparation, can solve the problem of amino acid combination that has not been seen, and achieve the purpose of increasing oral bioavailability and reducing toxic and side effects , the effect of promoting absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Embodiment 1: the synthesis of paliperidone glycinate (compound 1)
[0015] a. Protection of amino groups
[0016] Add 0.75g (10mmol) of L-glycine into the flask, add 10ml of water, 20ml of acetone, and add 2.08ml of triethylamine (Et 3 N), control the temperature and add di-tert-butyl dicarbonate (Boc) under stirring at 25°C 2 O 4.27ml (20mmol), and continue to stir for 4.5h, distill the acetone off under reduced pressure, extract the water layer 3 times with diethyl ether, 10ml each time, adjust the pH value of the water layer to 2-3 with dilute HCl, then dilute the water layer with ethyl acetate Extract 3 times, 15ml each time, combine the ethyl acetate layers, wash 2 times with saturated brine, 10ml each time, dry over anhydrous sodium sulfate, filter and evaporate to dryness, the product obtained is washed with ethyl acetate and petroleum ether (1:2 , volume ratio) to crystallize to obtain Boc-L-glycine with a yield of 90%. (Yield rate: the ratio of the actual p...
Embodiment 2
[0021] Embodiment 2: the synthesis of paliperidone DL-alanine ester (compound 2)
[0022] a. Protection reaction of amino group:
[0023] Add DL-alanine 0.89g (10mmol) into the flask, dissolve in 3.5ml of water and 7ml of 1,4-dioxane, cool with ice water, add dropwise 2.2ml of di-tert-butyl dicarbonate ( 11mmol) in 1,4-dioxane (7ml) and sodium hydroxide (0.5g) in water (7ml), after the dropwise addition was completed, the mixture was stirred in ice bath for 2h and at room temperature overnight. Add 1 mol / l hydrochloric acid to adjust the pH to 2, extract 3 times with ethyl acetate, combine, wash with 1 mol / l hydrochloric acid and water three times, dry over anhydrous sodium sulfate, evaporate the solvent, and recrystallize petroleum ether to obtain a white solid , the yield was 70%.
[0024] b. Synthesis of esters:
[0025] Dissolve 3mmol of protected alanine in 20ml of anhydrous dichloromethane, add catalyst DMAP, add 3mmol of paliperidone under stirring, add 10mmol of DCC...
Embodiment 3
[0028] Embodiment 3: the synthesis of paliperidone D-valine ester (compound 3)
[0029] a. Protection of amino groups
[0030] Add D-valine (10mmol) into the flask, add 10ml of water, 20ml of acetone, and add 2.08ml of Et 3 N, control the temperature and add (Boc) under stirring at 25°C 2 O (10mmol, 2.2ml), and continued to stir for 4.5h, distilled off the acetone under reduced pressure, extracted the water layer with diethyl ether 3 times, 10ml each time, adjusted the pH value of the water layer to 2-3 with dilute HCl, and then Esters were extracted 3 times, 15ml each time, the organic layers were combined, washed 2 times with saturated brine, 10ml each time, anhydrous Na 2 SO 4 After drying, it was filtered and evaporated to dryness, and the obtained product was crystallized with ethyl acetate and petroleum ether (1:2, volume ratio) to obtain Boc-D-valine with a yield of 70%.
[0031] b. Synthesis of esters:
[0032] The D-valine after the protection of 2.6mmol and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com