Felodipine solid dispersion (SD) and preparation method thereof
A technology of solid dispersion and dipine solid state, which is applied in the direction of non-active ingredient medical preparations, drug combinations, pharmaceutical formulas, etc., can solve the problems of complex preparation process, cumbersome production process, and no description of dissolution results, etc., and achieve simple preparation process , the effect of easy-to-control preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
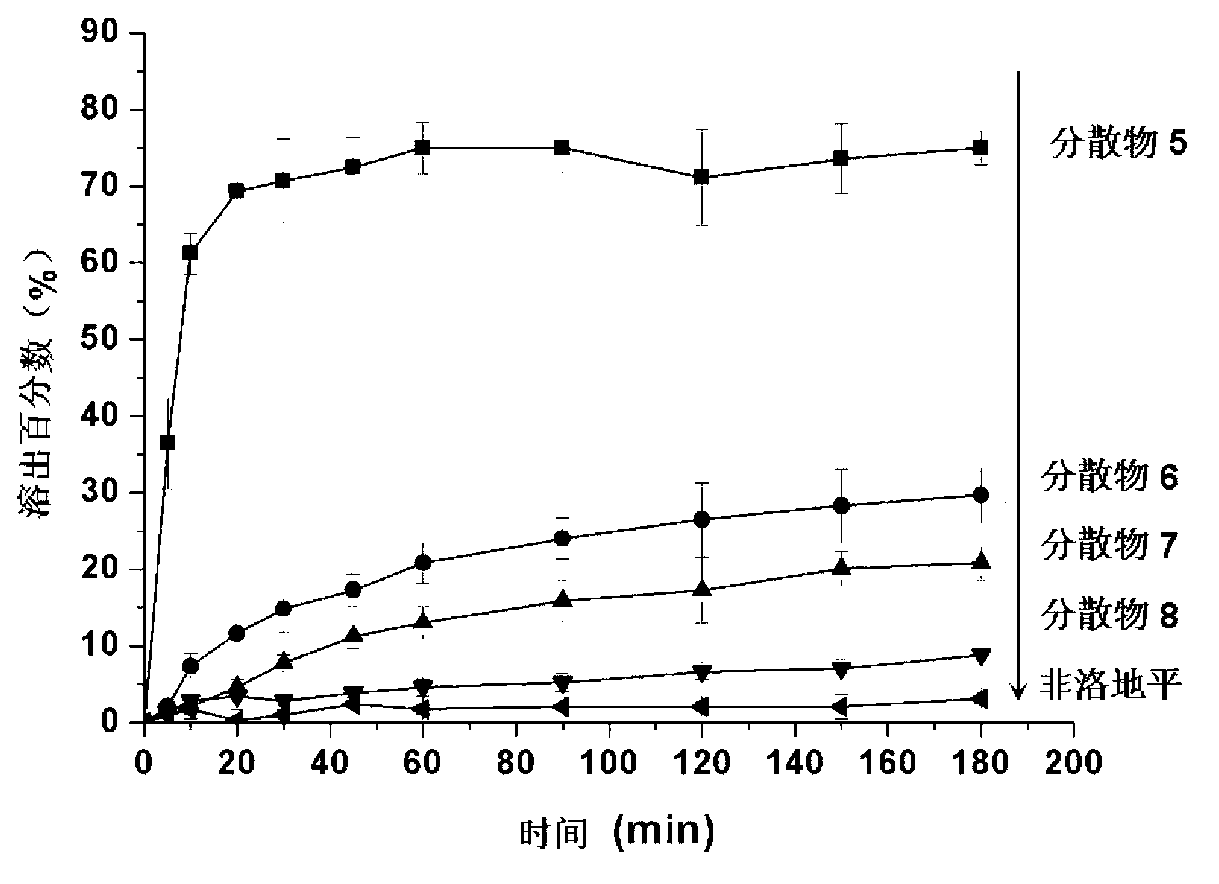
[0023] The embodiment of the present invention also discloses the preparation method of the above-mentioned felodipine solid dispersion, comprising: weighing and uniformly mixing the felodipine active drug and the solid dispersion carrier according to a certain mass ratio, and hot-melt extruding the mixture to obtain an extrudate , cooled to room temperature, and a solid dispersion was obtained after grinding.
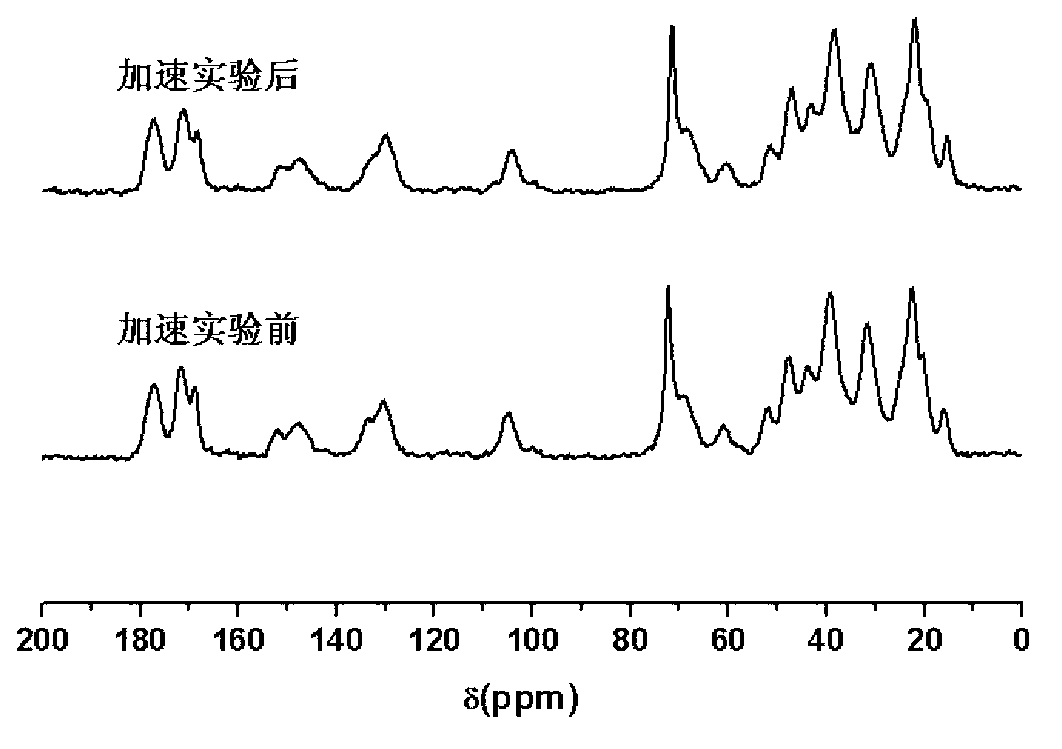
[0024] The present invention is further illustrated by the following examples: According to the following examples, the present invention can be better understood. However, those skilled in the art can easily understand that the specific material ratios, process conditions and results described in the examples are only used to illustrate the present invention, and should not and will not limit the present invention described in the claims.
Embodiment 1
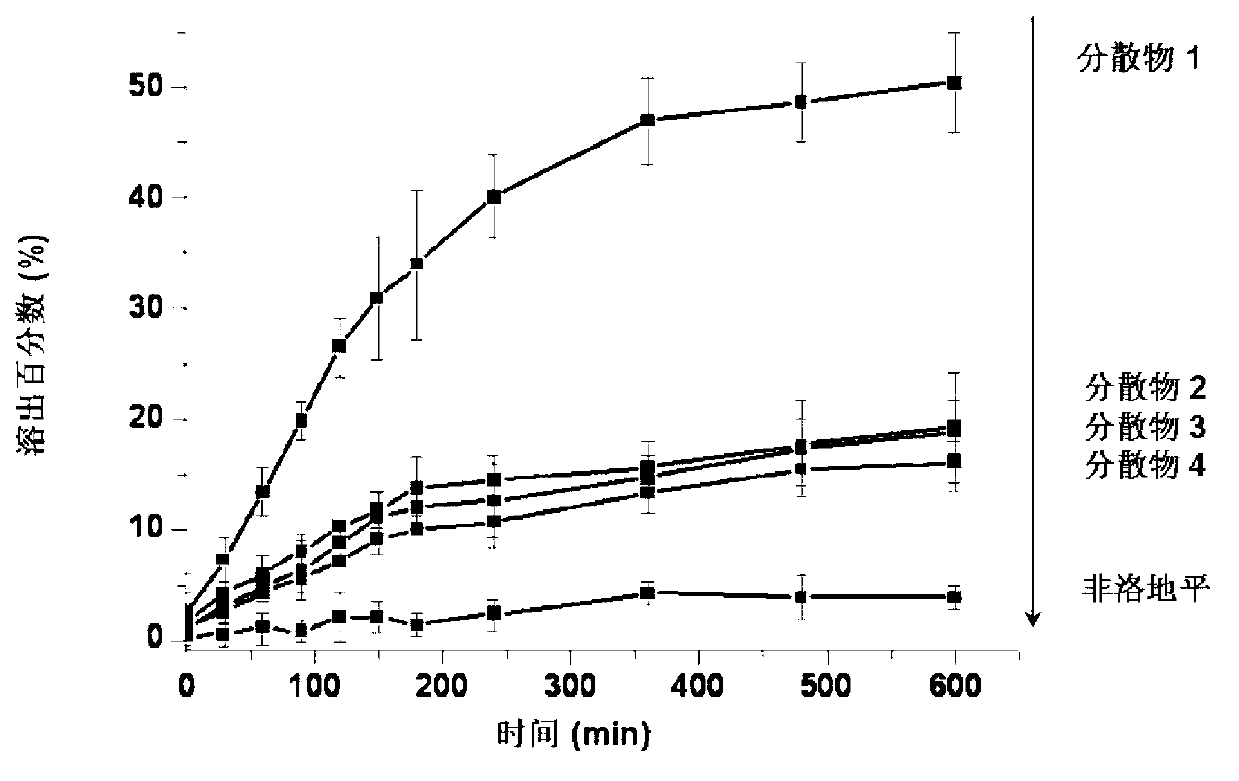
[0025] Embodiment 1: solid dispersion 1
[0026] Take by weighing 2 g of felodipine medicine and 18 g of solid dispersion carrier Soluplus, after uniform mixing, the mixture is fed into a hot-melt extruder at a suitable temperature, the screw speed of the extruder is: 20 rpm, and the extrusion temperature is: At 130 ℃, a transparent strip-shaped extrudate was obtained from the exit of the extruder, cooled to room temperature, crushed to a certain particle size according to subsequent preparation or analysis requirements, sieved, and collected a solid dispersion with a particle size of 75-250 μm.
Embodiment 2
[0027] Embodiment 2: solid dispersion 2
[0028] Weigh 4 g of felodipine drug and 16 g of solid dispersion carrier Soluplus, after uniform mixing, the mixture is fed into a hot-melt extruder, the extruder screw speed is: 20 rpm, extrusion temperature: 130 °C, Obtain a transparent strip extrudate from the exit of the extruder, cool to room temperature, crush to a certain particle size according to subsequent formulation or analysis requirements, sieve, and collect a solid dispersion with a particle size of 75-250 μm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com