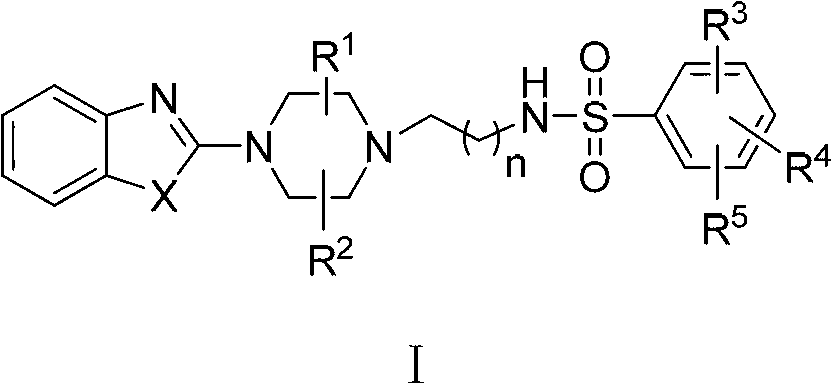
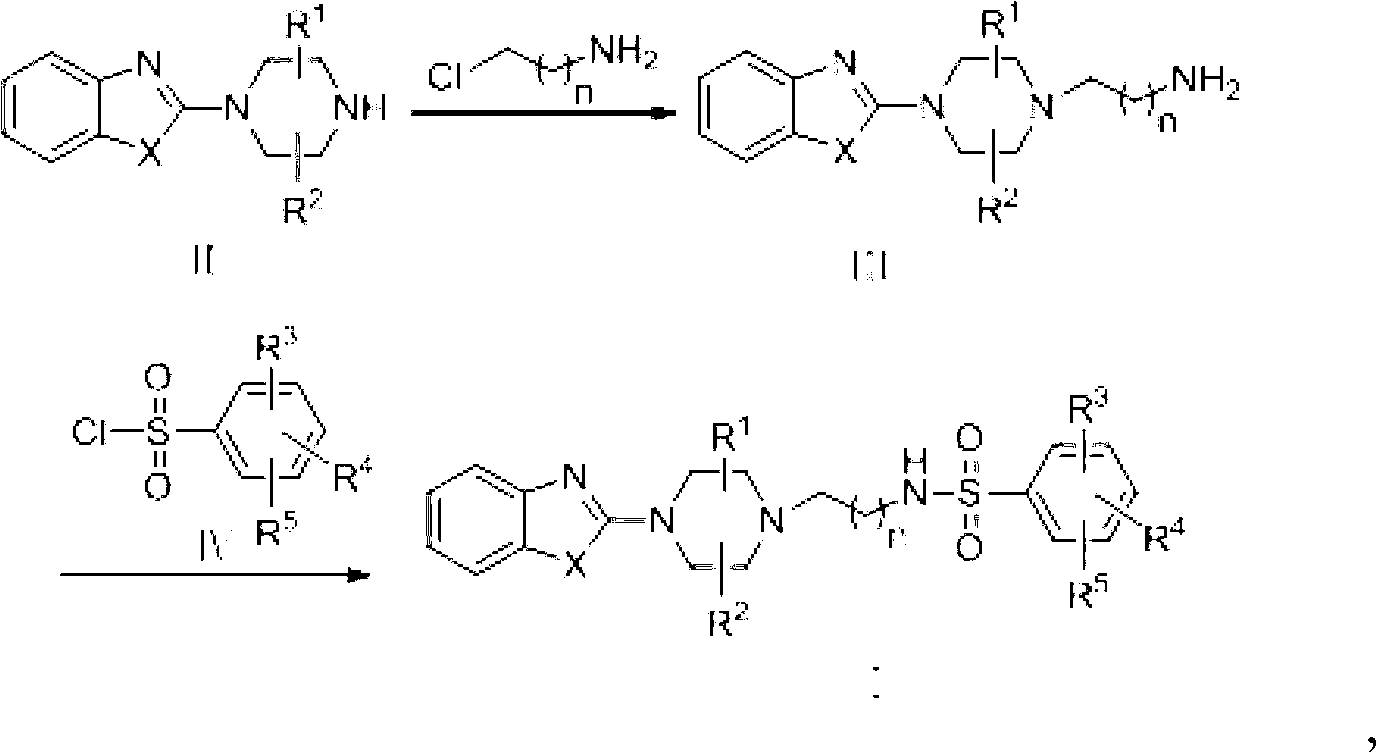
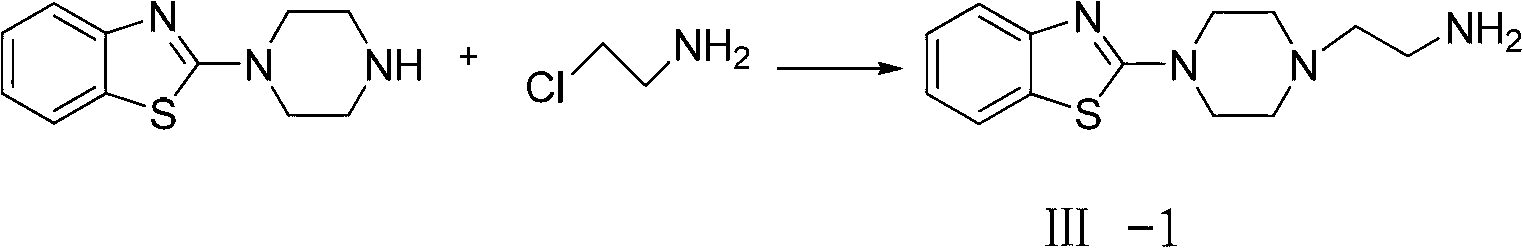
Sulfonamide compound and preparation method as well as application thereof
A compound, sulfonamide technology, applied in the field of medicine, can solve the problems of inability to maintain long-term efficacy, weight gain, hypoglycemia, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: the preparation of intermediate III
[0047] Take intermediate Ⅲ-1 as an example
[0048]
[0049]Add 2-(piperazin-1-yl)benzothiazole (2.19g, 0.01mol), 3-chloropropylamine (1.2g, 0.015mol) and sodium hydroxide into a reaction flask equipped with stirring, condenser and thermometer (7.2g, 0.18mol), dissolved in DMF (30ml), and reacted at 50°C for 4h. Naturally cool to room temperature, filter, add potassium acetate (0.24g) to the filtrate, vacuum distill about 25mL of DMF, add 50mL of water to the residue for dilution, adjust the pH value to 4.6 with acetic acid, dichloromethane extraction, the water layer with 12 % ammonia solution to adjust the pH value to 9.5, then extracted twice with dichloromethane, 50mL each time, dried over anhydrous sodium sulfate, and evaporated the solvent to obtain a yellow oily product as intermediate Ⅲ-1, HPLC: 92%, HRMS (m / z): 263.1325.
[0050] The compound can be conveniently prepared by referring to the method of Exa...
Embodiment 2
[0054] Preparation of N-(2-(4-(benzothiazol-2-yl)piperazin-1-yl)ethyl)-3,5-dimethylbenzenesulfonamide (compound Ⅰ-1):
[0055]
[0056] Add 2.46g (0.01mol) of the intermediate III-1 prepared in Example 1 into a reaction flask equipped with stirring, condenser and thermometer, dissolve it with 10mL of dichloromethane, stir, and add 2.2g of anhydrous potassium carbonate , add 2.1g (0.01mol) of 3,5-dimethylphenyl-1-sulfonyl chloride dropwise under ice-bath conditions, after dropping, stir at room temperature for about 3h, TLC detects that the reaction is complete, filter the solid, and wash the filtrate three times with water , water 15mL each time, the organic layer was fully dried with anhydrous sodium sulfate, filtered, and the organic solvent was evaporated under reduced pressure to obtain a light yellow oil. The white solid product obtained by silica gel column chromatography was compound Ⅰ-1 (HPLC: 99.5%) . Melting point 161°C-162.1°C, HRMS (m / z): 431.1570.
[0057] Co...
Embodiment 3
[0066] Formation of compound I-10 into hydrochloride: take 2.0 g of the white solid product of compound I-10 and dissolve it in 10 mL of anhydrous ether. Cool in an ice-water bath to 0°C, add dropwise 25% diethyl ether solution of hydrochloric acid until the pH value is 2, and continue to stir for about 1 h in an ice-water bath. After filtration, a white solid was obtained, which was the hydrochloride of compound Ⅰ-10.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com