Device and method for performing aromatic heterocyclic lithium halide exchange reaction at room temperature
An aromatic heterocycle, lithium halide exchange technology, applied in chemical instruments and methods, chemical/physical/physical chemical processes, organic chemistry, etc. Universality, the effect of increasing the reaction temperature and shortening the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
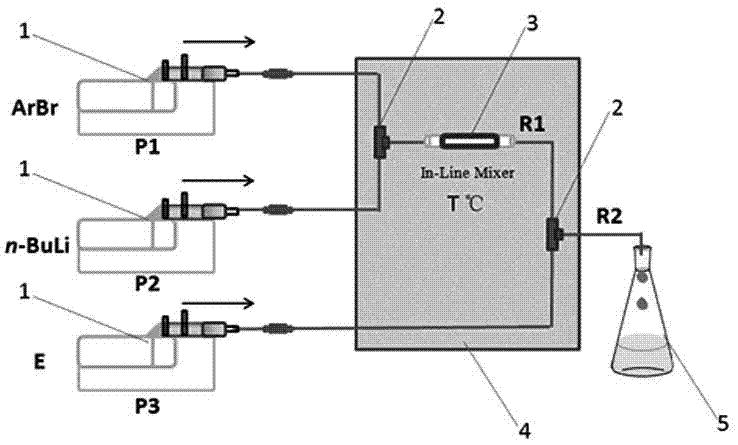
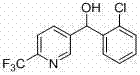
[0022] The microreactor was 500 microns internal diameter as previously described, the R1 reaction section was 20 cm, and the R2 reaction section was 150 cm. Take the raw material 5-bromo-2-trifluoromethylpyridine (362 mg, 1.6 mmol) and dissolve it in 10 mL tetrahydrofuran, draw it into a 10 mL syringe and connect it to the microautosampler P1, n-butyllithium solution (1 mL, 1.6 M ) was diluted to 0.16 M with n-hexane and drawn into a 10 mL syringe connected to a micro-volume autosampler device P3. After connecting the syringes, start the micro-injection pumps one by one, uniformly control the flow rate to 2.7 mL / min (the reaction retention time is 1 second), P1 and P2 respectively drive 5-bromo-2-trifluoromethylpyridine and n-butyllithium online After the reaction in the mixer is complete, the electrophilic substitution with P3-driven 2-chlorobenzaldehyde is completed to obtain the product. The reaction microtube was placed in a constant temperature bath at 0 °C, and the re...
Embodiment 2
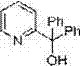
[0027]The microreactor was 500 microns internal diameter as previously described, the R1 reaction section was 20 cm, and the R2 reaction section was 200 cm. Take the raw material 5-bromo-2-methoxypyridine (300 mg, 1.6 mmol) and dissolve it in 10 mL tetrahydrofuran, draw it into a 10 mL syringe and connect it to the micro autosampler P1, n-butyllithium solution (1 mL, 1.6 M) Dilute to 0.16 M with n-hexane and draw into a 10 mL syringe connected to micro autosampler P2, dissolve benzophenone (437 mg, 2.4 mmol) in 10 mL THF and draw into a 10 mL syringe connected to micro autosampler P3 . After connecting the syringes, start the micro-injection pumps in sequence, uniformly control the flow rate to 2.7 mL / min (reaction retention time is 1 second), P1 and P2 respectively drive 5-bromo-2-methoxypyridine and n-butyllithium to mix online After the reaction in the reactor is complete, the electrophilic substitution with P3-driven benzophenone is completed to obtain the product. The r...
Embodiment 3
[0032] The microreactor was 500 microns internal diameter as previously described, the R1 reaction section was 20 cm, and the R2 reaction section was 200 cm. Take the raw material 2-bromopyridine (252 mg, 1.6 mmol) and dissolve it in 10 mL tetrahydrofuran, draw it into a 10 mL syringe and connect it to the micro autosampler P1, and dilute the n-butyllithium solution (1 mL, 1.6 M) with n-hexane to 0.16 M was drawn into a 10 mL syringe connected to micro autosampler P2, methyl 2-methoxybenzoate (398 mg, 2.4 mmol) was dissolved in 10 mL THF and drawn into a 10 mL syringe connected to micro autosampler P3. After connecting the syringes, start the micro-injection pumps one by one, uniformly control the flow rate to 2.7 mL / min (the reaction retention time is 1 second), P1 and P2 respectively drive 2-bromopyridine and n-butyllithium to complete the reaction in the online mixer, Complete electrophilic substitution with P3-driven methyl 2-methoxybenzoate affords the product. The react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com