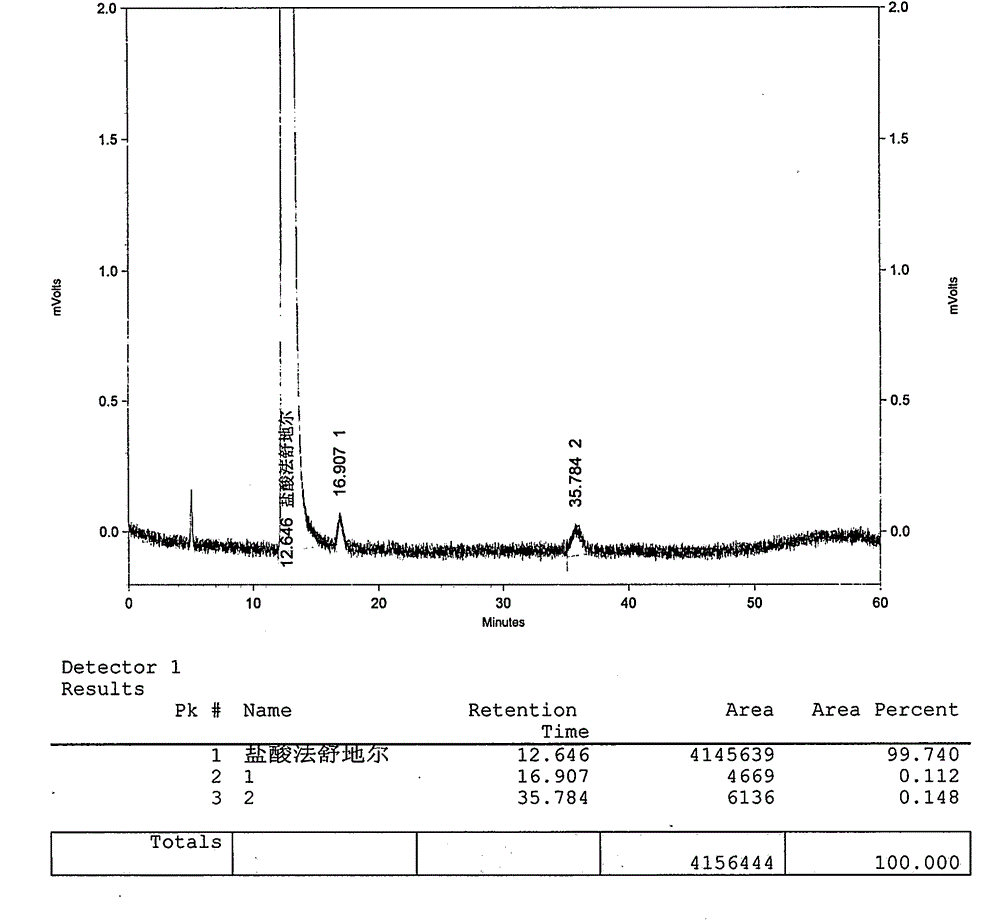
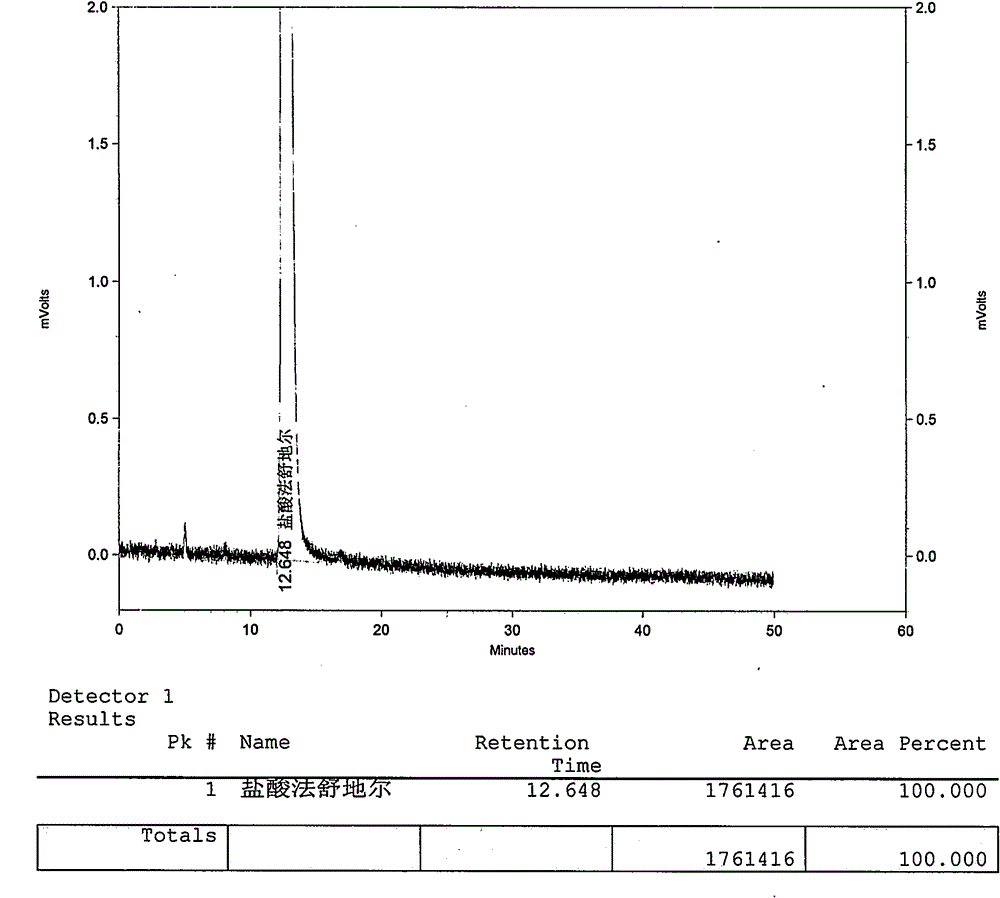
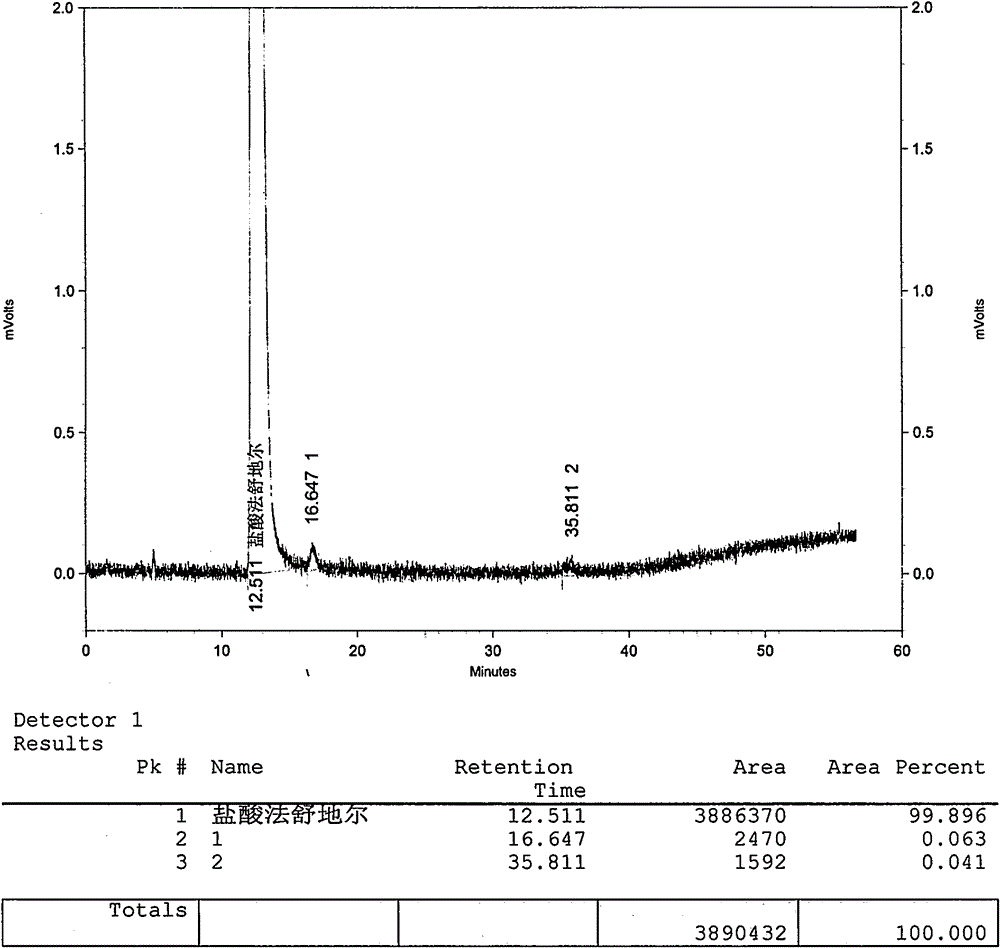
Method for preparing fasudil hydrochloride
A technology of fasudil hydrochloride and sulfonyl chloride hydrochloride, which is applied in the field of medicine and achieves the effects of high yield, controllable quality and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The preparation of embodiment 1 Fasudil hydrochloride
[0038] (1) Add isoquinoline-5-sulfonic acid (60g, 0.287mol) into a 1000mL three-necked flask, add thionyl chloride (500ml, 6.893mol), and add 4mL of N,N-dimethylformamide dropwise , mechanically stirred, and heated to reflux for 6h. Generate hydrogen chloride and sulfur dioxide gas with 40% sodium hydroxide solution to absorb. Lower the temperature slightly, and distill off excess thionyl chloride under reduced pressure. When the temperature was lowered to room temperature, 160 mL of dichloromethane was added to the reaction flask and stirred for 30 minutes, cooled to below 20°C, filtered with suction, the filter cake was washed three times with dichloromethane, and dried under reduced pressure to obtain 61.9 g of white solid powder isoquinoline-5 -sulfonyl chloride hydrochloride.
[0039] (2) Add 454 mL each of water and dichloromethane to a 2000 mL three-necked flask, cool down to about 0°C, add isoquinoline-5...
Embodiment 2
[0042] The preparation of embodiment 2 Fasudil hydrochloride
[0043] (1) Add 0.6kg of isoquinoline-5-sulfonic acid and 5L of thionyl chloride into a 20L reactor, stir mechanically, then add 40mL of DMF, and reflux for 4.5 to 6 hours. After the reaction is completed, the temperature of the reaction solution is lowered to about 50-55°C, and after the thionyl chloride is evaporated under reduced pressure, the temperature is lowered to 20-30°C, and 1.6L of CH 2 Cl 2 , stirred, suction filtered, the filter cake was washed with dichloromethane, and dried under reduced pressure to obtain 0.705kg of isoquinoline-5-sulfonyl chloride hydrochloride.
[0044] (2) Add 4.5L of water and 4.5L of methylene chloride in a 20L reactor, stir mechanically, add 0.705kg of isoquinoline-5-sulfonyl chloride hydrochloride and 0.2kg of NaHCO 3 , the temperature is controlled not to exceed 10°C, and the pH value is adjusted to 7. After stirring and reacting for 30 minutes, let stand to separate layer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com