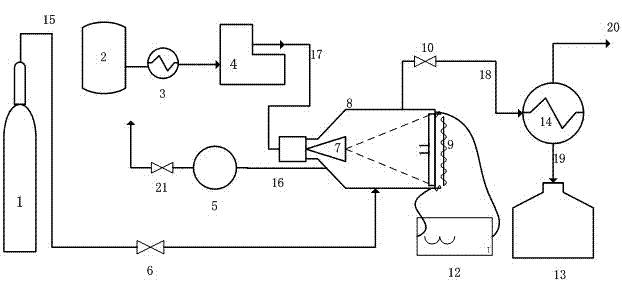
Method and device for catalytic preparation of hydrogen from naphthenic hydrocarbon
A cycloalkane and catalyst technology, which is applied in the field of hydrogen production from organic liquids, can solve the problems of unsuitable large-scale hydrogen production, bulky reaction device, and poor reaction continuity, so as to improve the dehydrogenation reaction efficiency and improve the reaction efficiency Good efficiency and catalytic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Take 3 g of catalyst Raney nickel, wash 3 times with absolute ethanol to replace the water therein, and press the catalyst into a circular sheet, then add it to a 50ml reactor and fix it at the bottom of the reactor; turn on the vacuum pump Vacuumize the reactor, turn off the vacuum pump, open the nitrogen bottle, feed nitrogen into the reactor, heat the bottom of the reactor under the protection of nitrogen, and slowly raise the temperature of the catalyst at a rate of 10°C / min to remove the combined catalyst in Raney nickel. Water and absolute ethanol, when the temperature rises to 160°C, the catalyst has been dried, at this time, the nitrogen flow is stopped, and the organic liquid condenser is opened to cool the cyclohexane to 6°C; the dehydrogenation reaction temperature is set to 326°C, and the reaction The bottom of the reactor was heated to the dehydrogenation reaction temperature. After the catalyst temperature was stabilized, the syringe pump was turned on, and...
Embodiment 2
[0026] Take 4.6g of catalyst Raney nickel, wash 3 times with absolute ethanol to replace the moisture therein, and press the catalyst into a circular sheet, then add it to a 50ml reactor, and fix it at the bottom of the reactor; turn on the vacuum pump Vacuumize the reactor, turn off the vacuum pump, open the nitrogen bottle, feed nitrogen into the reactor, heat the bottom of the reactor under the protection of nitrogen, and slowly raise the temperature of the catalyst at a rate of 10°C / min to remove the combined catalyst in Raney nickel. Water and absolute ethanol, when the temperature rises to 160°C, the catalyst has been dried, at this time, the nitrogen flow is stopped, and the organic liquid condenser is opened to cool the methylcyclohexane to 10°C; the dehydrogenation reaction temperature is set to 315°C, and The bottom of the reactor was heated to the dehydrogenation reaction temperature. After the catalyst temperature was stabilized, the syringe pump was turned on. Meth...
Embodiment 3
[0028] Take 7 g of catalyst Raney nickel (apparent density is about 3.0 g cm -3, with an average particle size of 16.58 μm), washed three times with absolute ethanol to replace the water in it, and pressed the catalyst into a circular thin layer, then added it to a 50ml reactor and fixed it at the bottom of the reactor; turned on the vacuum pump to drain the reactor Vacuumize, turn off the vacuum pump, open the nitrogen bottle, feed nitrogen into the reactor, heat the bottom of the reactor under the protection of nitrogen, and slowly raise the temperature of the catalyst at a rate of 10°C / min to remove bound water and free Water ethanol, when the temperature rises to 160°C, the catalyst has been dried, at this time stop nitrogen, open the organic liquid condenser to cool decahydronaphthalene to 2°C; set the dehydrogenation reaction temperature to 346°C, and heat the bottom of the reactor To the dehydrogenation reaction temperature, after the catalyst temperature is stabilized,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com