Photochemical synthesis method of 25-hydroxy vitamin D3
A technology for hydroxyvitamin and photochemical synthesis, which is applied in the field of photochemical synthesis of 25-hydroxyvitamin D3, can solve the problems of difficult separation, low total yield, many by-products, etc., and achieves the improvement of reactant concentration, utilization efficiency, and simple production process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
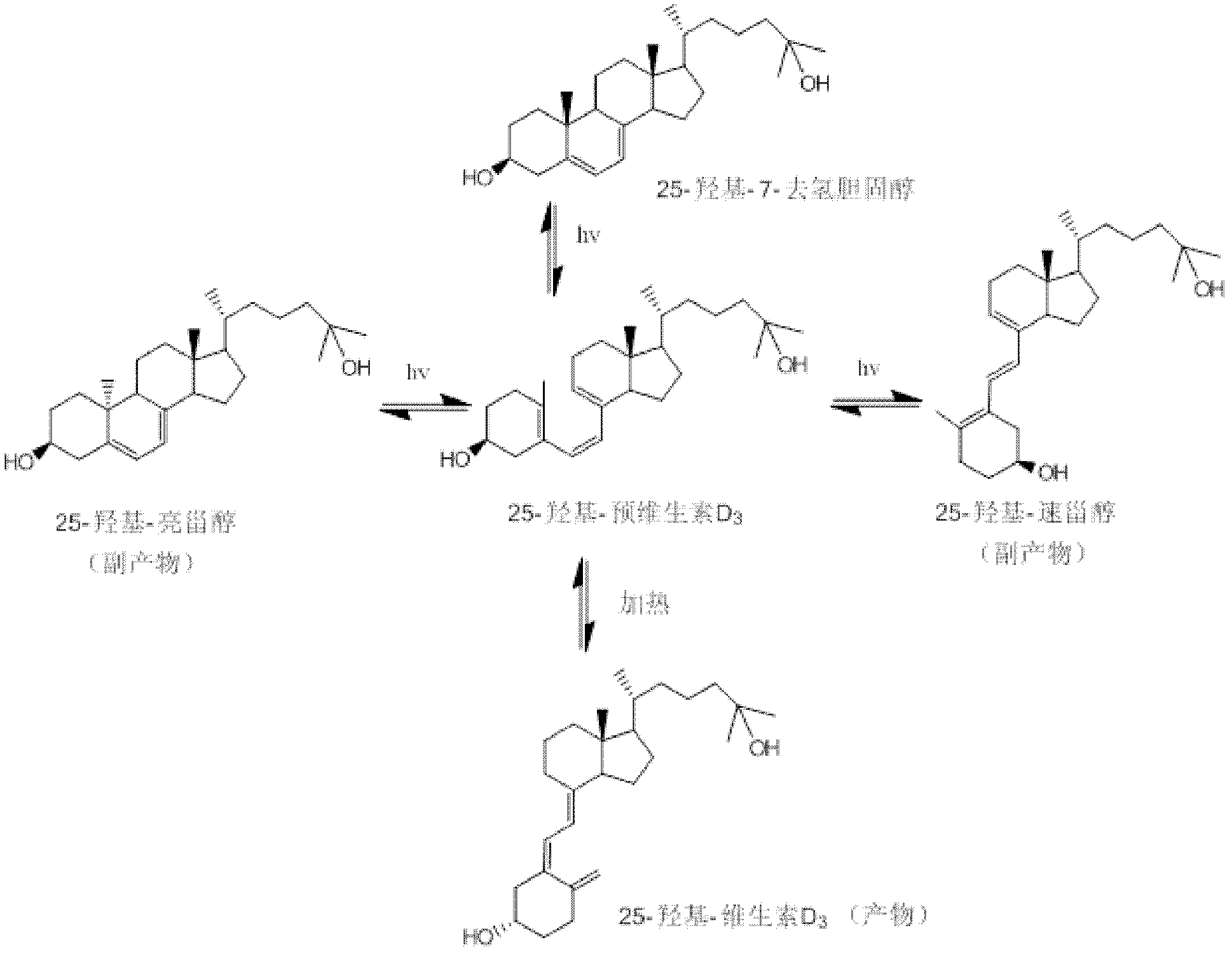
[0045] Photochemical synthesis of 25-hydroxyvitamin D 3 method, including the following steps:
[0046] 1) Light reaction of 25-hydroxy-7 dehydrocholesterol
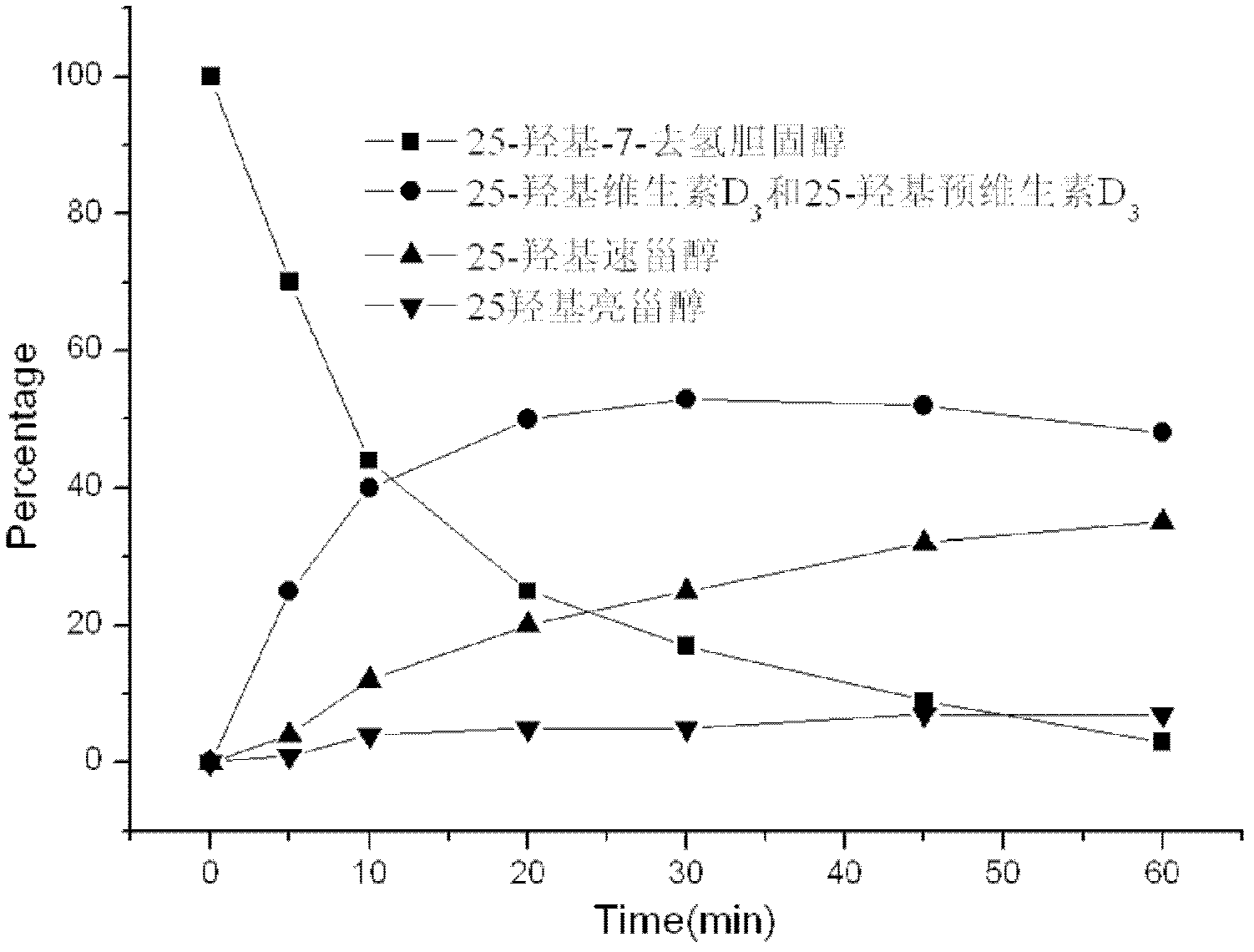
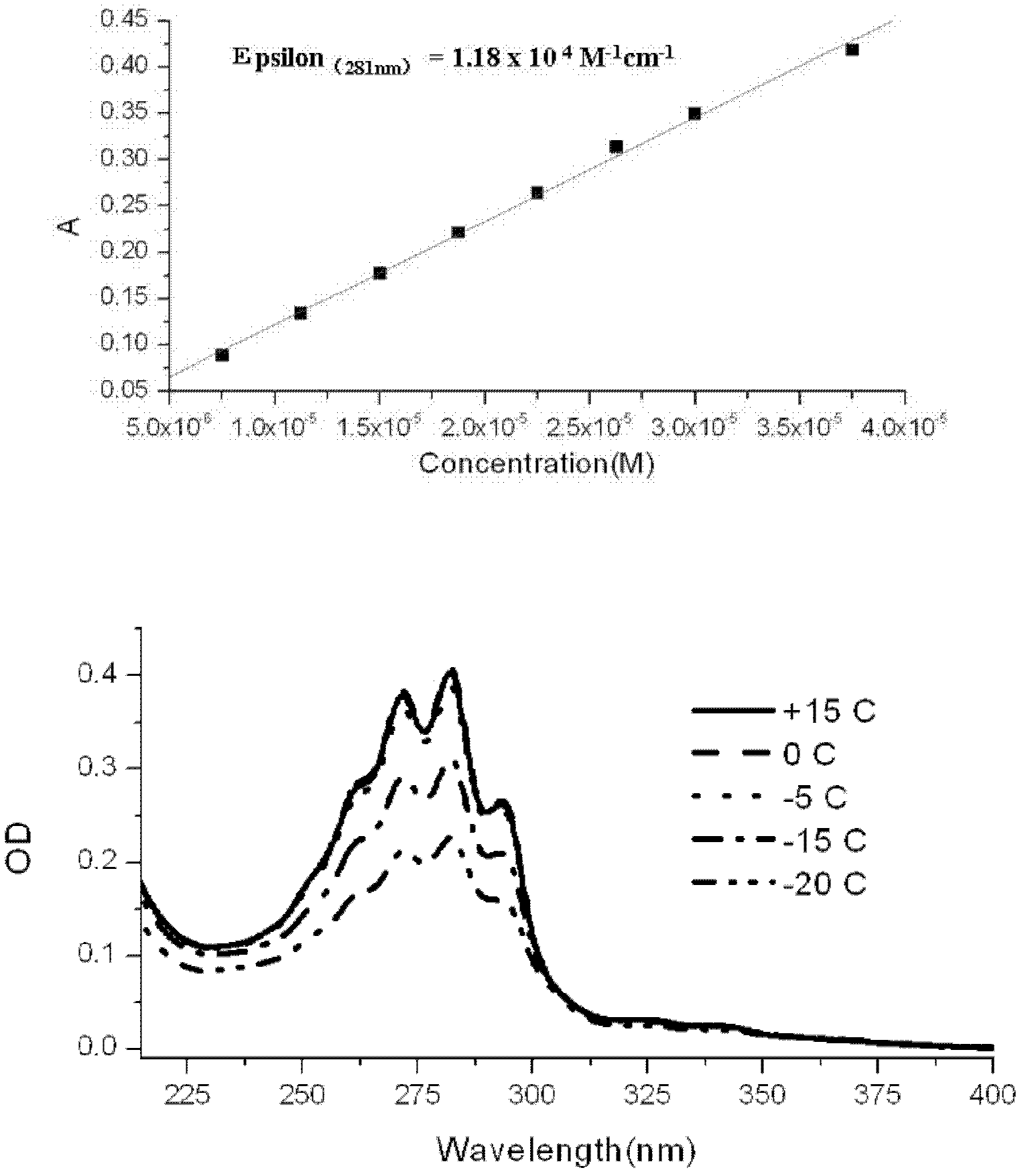
[0047] In a 500 ml round bottom flask, dissolve 10 g of 25-hydroxy-7 dehydrocholesterol in 280 ml of ethanol / n-pentane (6:1, V / V) mixed solvent at room temperature, add 20 mg of 2,6 -Di-tert-butyl-p-methoxyphenol, mixed evenly, configured into a photochemical reaction solution; the reaction solution was placed in a built-in photochemical reactor with a 500W high-pressure mercury lamp and nitrogen gas; adjust the flow rate of nitrogen and Condensed water flow; start the mercury lamp, and time; the entire photochemical reaction device is placed in a light-proof fume hood, and a small fan is used to promote the dispersion of radiant heat to ensure that the temperature of the photochemical reaction liquid does not exceed 25 ° C to avoid premature generation 25-Hydroxyvitamin D 3 ; Monitor the reaction with high performanc...
Embodiment 2
[0053] Photochemical synthesis of 25-hydroxyvitamin D 3 method, including the following steps:
[0054] 1) Light reaction of 25-hydroxy-7 dehydrocholesterol
[0055] In a 500 ml round bottom flask, 10 g of 25-hydroxy-7 dehydrocholesterol was dissolved in 280 ml of isopropanol / n-octane (9:1, V / V) mixed solvent at room temperature, and 20 mg of 2 , 6-di-tert-butyl-p-methoxyphenol, mixed evenly, and configured as a photochemical reaction solution; put the reaction solution into a built-in photochemical reactor with a 500W high-pressure mercury lamp and nitrogen gas; adjust the nitrogen gas The flow rate and condensed water flow rate; start the mercury lamp, and time; the entire photochemical reaction device is placed in a light-proof fume hood, and a small fan is used to promote the dispersion of radiant heat to ensure that the temperature of the photochemical reaction solution does not exceed 25 ° C, avoid Premature production of 25-hydroxyvitamin D 3 ; Monitor the reaction w...
Embodiment 3
[0061] Photochemical synthesis of 25-hydroxyvitamin D 3 method, including the following steps:
[0062] 1) Light reaction of 25-hydroxy-7 dehydrocholesterol
[0063] In a 500 ml round bottom flask, 10 g of 25-hydroxyl-7 dehydrocholesterol was dissolved in 280 ml of methanol / n-hexane (4:1, V / V) mixed solvent at room temperature, and 20 mg of 2,6- Di-tert-butyl-p-methoxyphenol, mixed evenly, and configured as a photochemical reaction solution; the reaction solution was placed in a built-in photochemical reactor with a 500W high-pressure mercury lamp and nitrogen gas flow. Adjust the flow rate of nitrogen and condensed water; start the mercury lamp, and time; place the entire photochemical reaction device in a light-proof fume hood, and use a small fan to promote the dispersion of radiant heat to ensure that the temperature of the photochemical reaction solution does not exceed 25 ℃, to avoid premature formation of 25-hydroxyvitamin D 3 ; Monitor the reaction with high-perform...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| power | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com