A kind of anti-human multidrug resistance protein fab antibody and preparation method thereof
A multi-drug resistance and antibody technology, applied in the direction of anti-animal/human immunoglobulin, botanical equipment and methods, biochemical equipment and methods, etc., to achieve the effect of chemotherapy, small impact and fast metabolism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
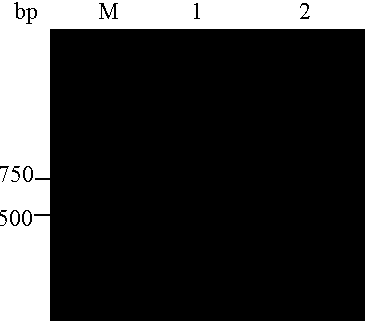
[0037] Cloning of P-gp transmembrane region gene sequence
[0038] 1. Design primers
[0039] Primers were designed with the following sequences:
[0040] Sense primer
CCGGAATTCCTCACCAAGCGGCTCCGAT
Anti-sense primer
CCGCTCGAGGAGTTTATGTGCCACCAAGTAG
[0041] Upstream (5′ end) primer introduction Eco RI restriction site, downstream (3' end) primer introduction xho Ⅰ Restriction sites, synthetic primers.
[0042] 2. Cloning of P-gp transmembrane region (amino acid 784-968) gene sequence
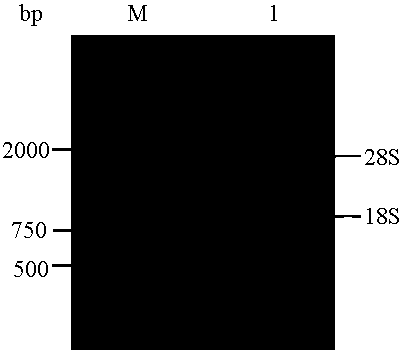
[0043] (1) Extract total RNA from multidrug-resistant human colorectal cancer tissue and reverse transcribe it into cDNA:
[0044] 1) Prepare 10 cases of multidrug-resistant colorectal cancer tissues to make colorectal cancer tissue homogenate for immunization; take the colorectal cancer tissue homogenate for immunization and inject subcutaneously into BALB / C mice at multiple points; if the antibody titer is greater than 1.512, it can be ;
[0045] 2) Extract ...
Embodiment 2
[0065] P-gp 784-968 gene expression
[0066] (1) Take the correctly identified positive clone P-gp 784-968 Inoculate 10 μL of the bacterial solution into 5 mL LB / Kan liquid medium, shake and cultivate overnight at 37°C and 220 rpm;
[0067] (2) Take 200 μL of the overnight culture and re-inoculate it into 20 mL of new LB / Kan liquid medium, culture at 37°C and shake at 220 rpm for about 4 hours, measure the OD 600nm About 0.4, take the bacteria solution;
[0068] (3) Then add IPTG (100mmol / mL) to the culture bottle to make the final concentration 0.5mmol / mL, continue shaking culture at 30°C and 220rpm for 3 hours, and take the bacterial liquid;
[0069] (4) Centrifuge the bacterial solution collected before and 3 hours after induction with IPTG at 12,000 rpm for 1 minute, discard the supernatant, and add 100 μL of PBS (pH: 7.4) to resuspend. Take 30 μL of bacterial solution and add 6 μL of 6× protein loading buffer, boil at 100°C for 10 minutes;
[0070] (5) Take 30 μL of ...
Embodiment 3
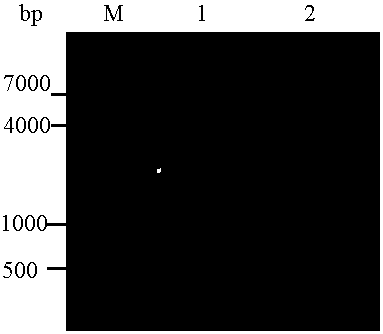
[0073] P-gp 784-968 Purification of expression products
[0074] (1) First, filter the lysate supernatant, triple-distilled water, Binding buffer, and each imidazole gradient Elute buffer with a 0.22 μm microporous filter; wash the nickel ion affinity chromatography column (Ni column) 5 volumes;
[0075] (2) Then the lysate supernatant flows through the nickel column at a flow rate of 1mL / min, washes 10 volumes of the nickel column with Binding buffer, and elutes the nickel column with Elute buffer with 50mM, 150mM, and 300mM imidazole concentrations respectively to obtain a purified sample , SDS-PAGE electrophoresis detection;
[0076] (3) P-gp 784-968 Dialysis and desalination of the purified sample: put the purified protein sample in a dialysis bag, close the dialysis bag and place it in PBS at 4°C for dialysis, replace the PBS every 12 hours, and continue dialysis for 36 hours, then take out the protein sample, Store at 4°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com