Ultrasonic biological effect mediated recombinant human endostatin controlled release preparation
A vascular endothelium and biological effect technology, which can be used in medical preparations containing active ingredients, drug combinations, non-active ingredients of polymer compounds, etc., can solve the problem of limited anti-tumor angiogenesis effect, etc. No toxic side effects, slow down the degradation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
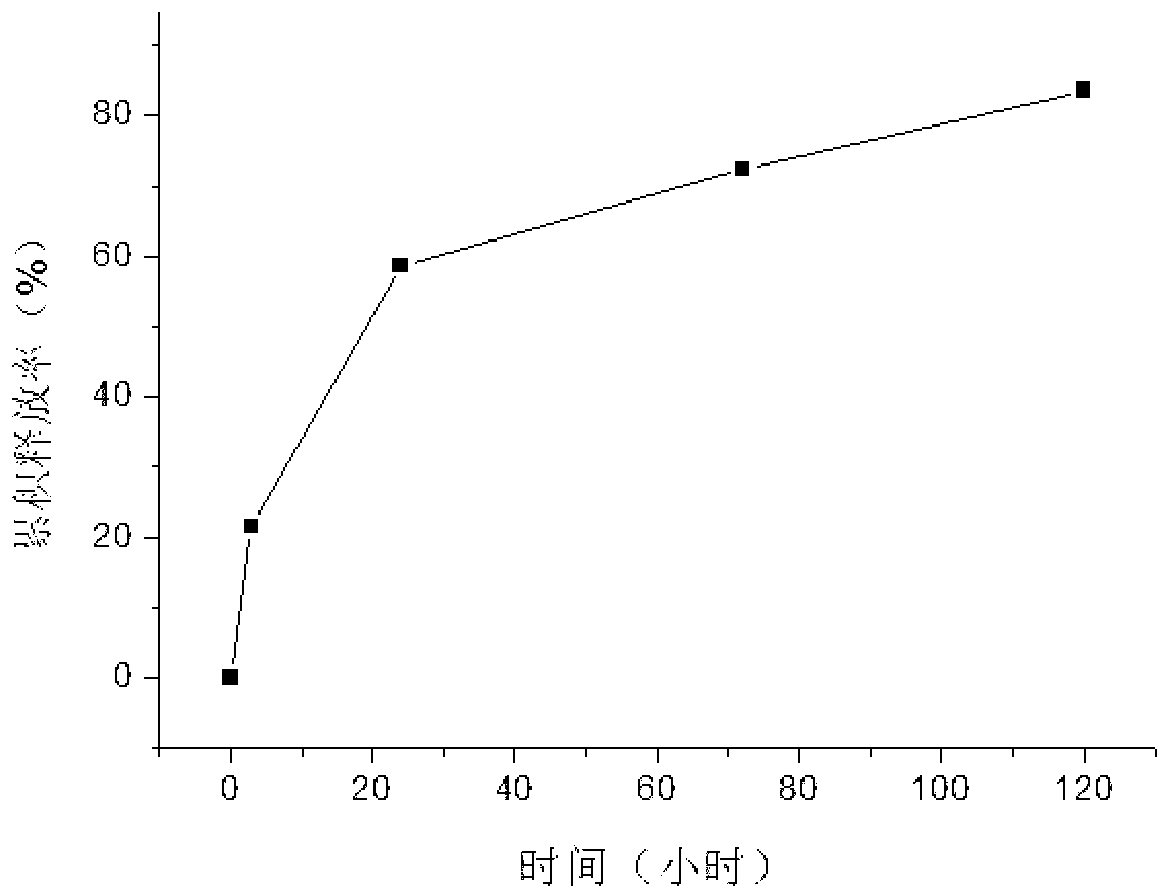
[0013] Take 2mg of rh-ES, add 1ml of medical biological protein glue (produced by Guangzhou Beixiu Biotechnology Co., Ltd.) main glue solution (fibrinogen concentration is 50mg / ml), mix well, and mix with 1ml of catalyst solution (containing 400IU thrombin) Mix to form drug-loaded biogel. Put the drug-loaded biogel in 10ml phosphate buffer (0.01mol / L, pH7.2, containing 0.2mg / ml NaN 3 ), in a constant temperature shaker at 37°C, 80 times / min, oscillating, for in vitro release, and ELISA to measure the released drug amount. The drug-loaded bioglue releases rh-ES more stably in vitro, 21.5% in 2 hours, and 89.7% of the cumulative drug release in 5 days; see figure 1 .
[0014] The above-mentioned medical biological protein glue: produced and provided by Guangzhou Beixiu Biotechnology Co., Ltd., is a commercially available product. Each set of "medical biological protein glue" includes the main body glue of medical biological protein glue (the present invention is formulated to...
Embodiment 2
[0016] The drug-loaded bioglue released in vitro was irradiated with ultrasound at a sound intensity of 1.0 W / cm 2 , after 3 minutes of irradiation, samples were taken once at 10 minutes and 30 minutes respectively, and the drug release was measured. It was found that ultrasonic irradiation significantly promoted the drug release rate of bioglue, and had the effect of controlling drug release.
[0017] Table 1 Effect of ultrasonic irradiation on drug release rate of drug-loaded biogel (%)
[0018] group 10min 30min control group 5.2±1.6 10.1±3.3 Ultrasound irradiation group 15.7±6.8 ** 19.5±7.5 **
[0019] Note: Compared with the control group, ** P < 0.01.
Embodiment 3
[0021] Human breast cancer cell MDA-MB-231 was cultured in vitro, amplified in large quantities, and subcutaneously inoculated into the root of the right forelimb of nude mice to establish a subcutaneous xenograft tumor model in nude mice bearing human breast cancer. Fifteen tumor-bearing nude mice were selected, with the largest tumor diameter ranging from 1.2cm to 1.5cm, and randomly divided into 3 groups, 5 mice in each group, respectively (group A) rh-ES biogel intratumoral injection group, (group B) Erh-ES Intratumoral injection of biological glue+ultrasound irradiation group, (Group C) intratumoral injection of rh-ES biological glue+microbubble contrast agent+ultrasound irradiation group. In group A, the drug-loaded biological glue was injected into the tumor, the dose of rh-ES was 10mg / kg, and the injection volume of biological glue was 0.5ml; 2 Tumor tissue was irradiated with ultrasound for 3 minutes; 10 minutes after intratumoral injection in nude mice in group C, ul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com