Polysaccharide acylate and preparation method thereof
A technology for acylate and polysaccharides, applied in the field of polysaccharide acylate and its preparation, can solve the problems of unfixed target structure, uncertain position of acylation, unstable activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
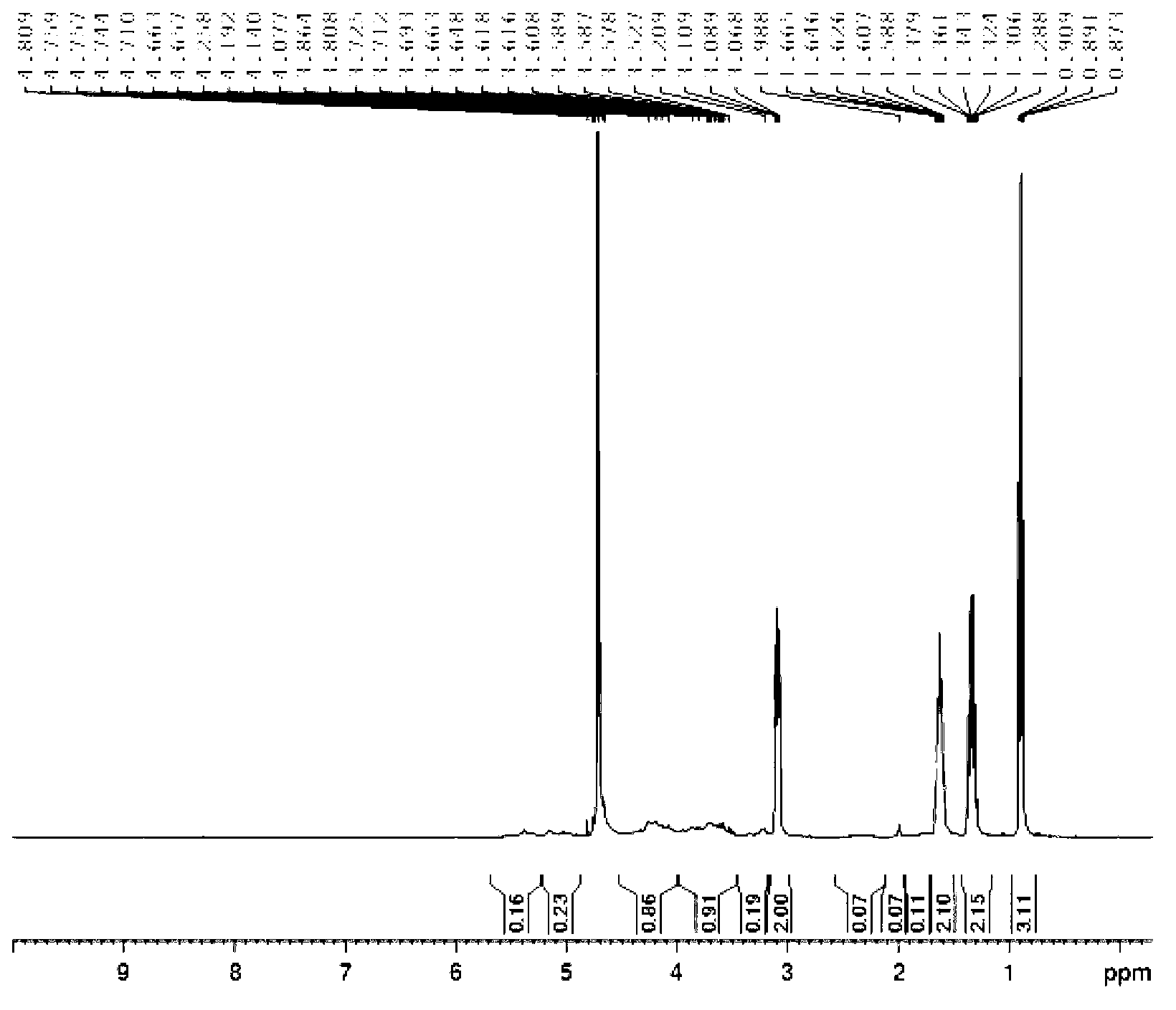
[0041] Dissolve 200mg of low-molecular-weight heparin sodium (Jiangsu Wanbang Biochemical Pharmaceutical Co., Ltd., molecular weight less than 8000Da) in 1-20ml of water, pass through an ion exchange column, collect the heparin aqueous solution, add 0.1-5.0ml of tributylamine, and freeze at -80°C for 1 hour , concentrated under reduced pressure to remove water to obtain 203 mg of solid. Then dissolve it in 2-50ml of dichloromethane solvent, add 1-100mg of DCC and stir to dissolve, add 1.1-2.0ml of butyric anhydride while stirring at 0°C, react for 1h, then stir at room temperature for 1-72h. Then add 1-50ml of 5% sodium hydroxide aqueous solution to the reaction solution, stir for 2-72h, filter to remove the solid, add dilute hydrochloric acid to adjust the pH value to 7, extract with ether to remove impurities, concentrate under reduced pressure and pass through a gel column to collect the target The product was dried under reduced pressure to obtain 150 mg of the light yello...
Embodiment 2
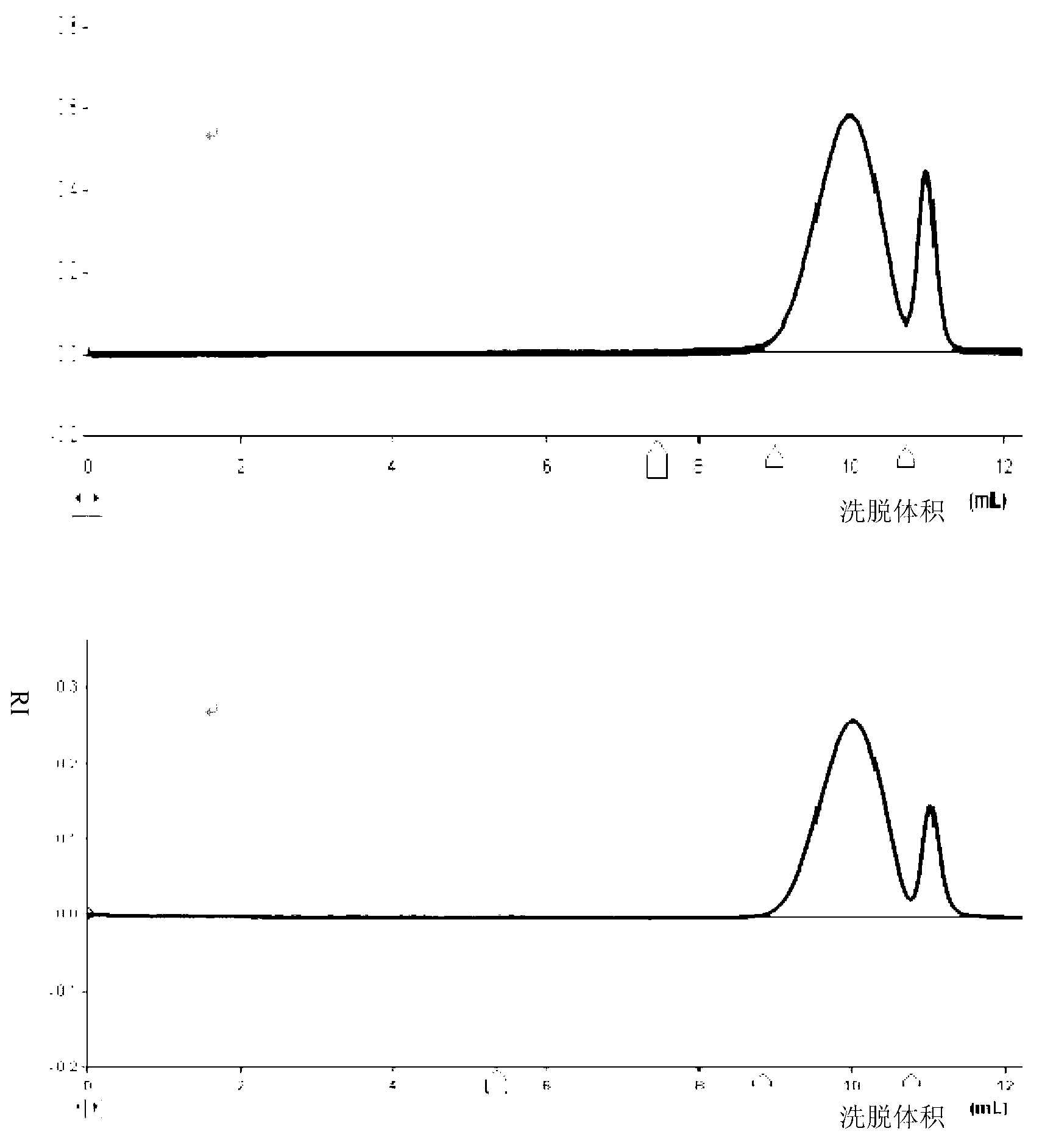
[0043] Dissolve 200 mg of low-molecular-weight heparin (Hebei Changshan Biochemical Pharmaceutical Co., Ltd., molecular weight less than 12,000 Da) in 1-20 ml of water, pass through an ion exchange column, collect the heparin aqueous solution, add 0.1-5 ml of tributylamine, and store at -80 ° C Freeze for 1 h, and concentrate under reduced pressure to remove water to obtain 203 mg of white solid. Then dissolve it in 2-50ml of dichloromethane solvent, add 1-100mg of DCC, stir to dissolve, add 0.1-1.0ml of butyric anhydride while stirring at 0°C, react for 1h, then stir at room temperature for 1-72h. Then add 1-50ml of 5% sodium hydroxide aqueous solution to the reaction solution, stir for 2-72h, filter to remove the solid, add dilute hydrochloric acid to adjust the pH value to 7, extract with ether to remove impurities, concentrate under reduced pressure and pass through a gel column to collect the target The product was dried under reduced pressure to obtain 160 mg of the ligh...
Embodiment 3
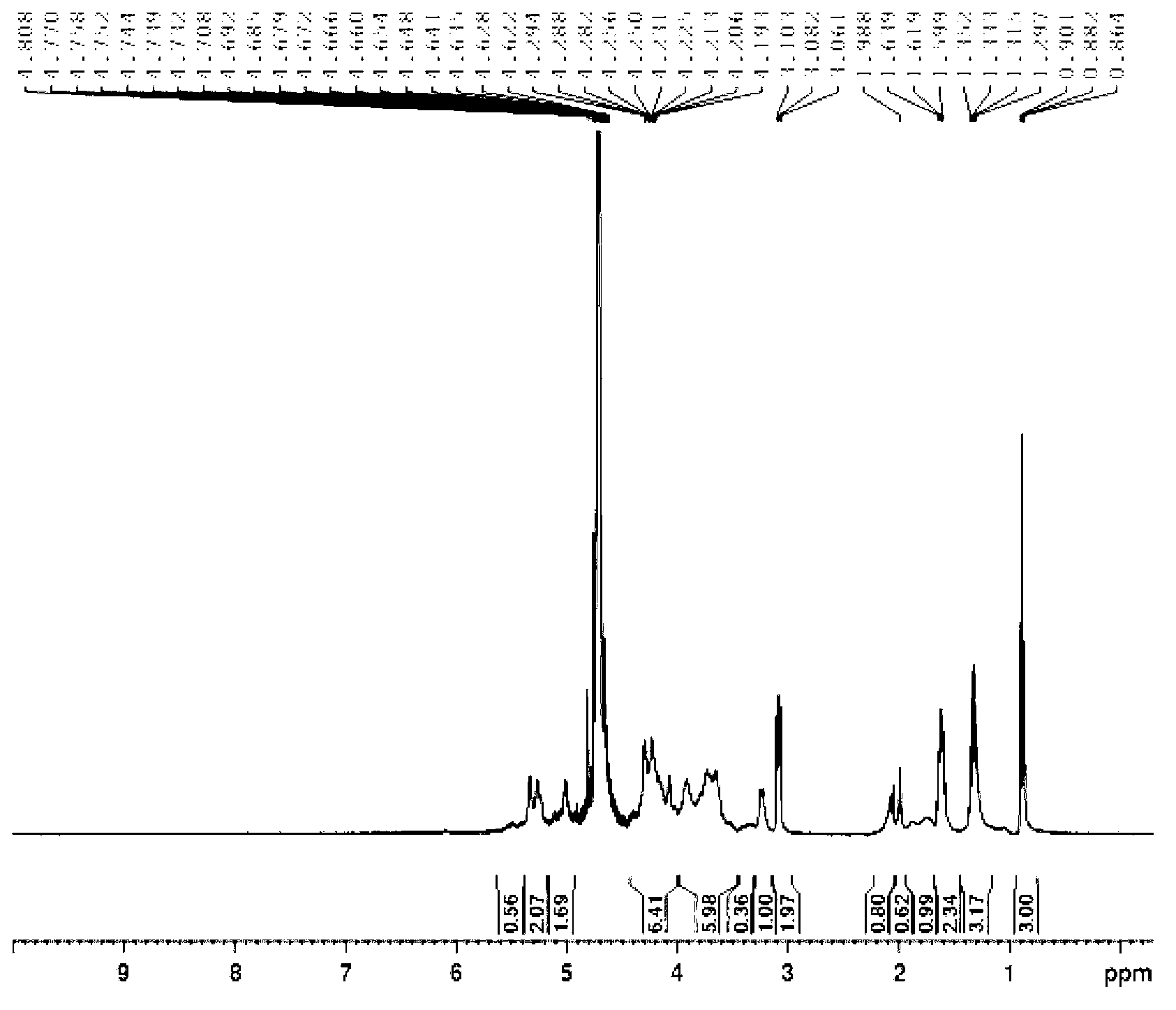
[0045] Dissolve 200mg of low-molecular-weight heparin sodium (self-degradation, molecular weight less than 4000Da) in 1-20ml of water, pass through an ion exchange column, collect the heparin aqueous solution, add 0.1-5ml of tributylamine, freeze at -80°C for 1 hour, and concentrate under reduced pressure to remove water 203mg of white solid was obtained. Then dissolve it in 2-50ml of dichloromethane solvent, add 1-100mg of DCC, stir to dissolve, add 2.1-5.0ml of acetic anhydride while stirring at 0°C, react for 1h, then stir at room temperature for 1-72h. Then add 1-50ml of 5% aqueous sodium hydroxide solution to the reaction solution, stir for 2-72h, add dilute hydrochloric acid to adjust the pH value to 7, dialyze for 2-72h, filter to remove impurities, freeze-dry to obtain 120mg of the light yellow target substance, (product rate 60%). 1 See HNMR image 3 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com