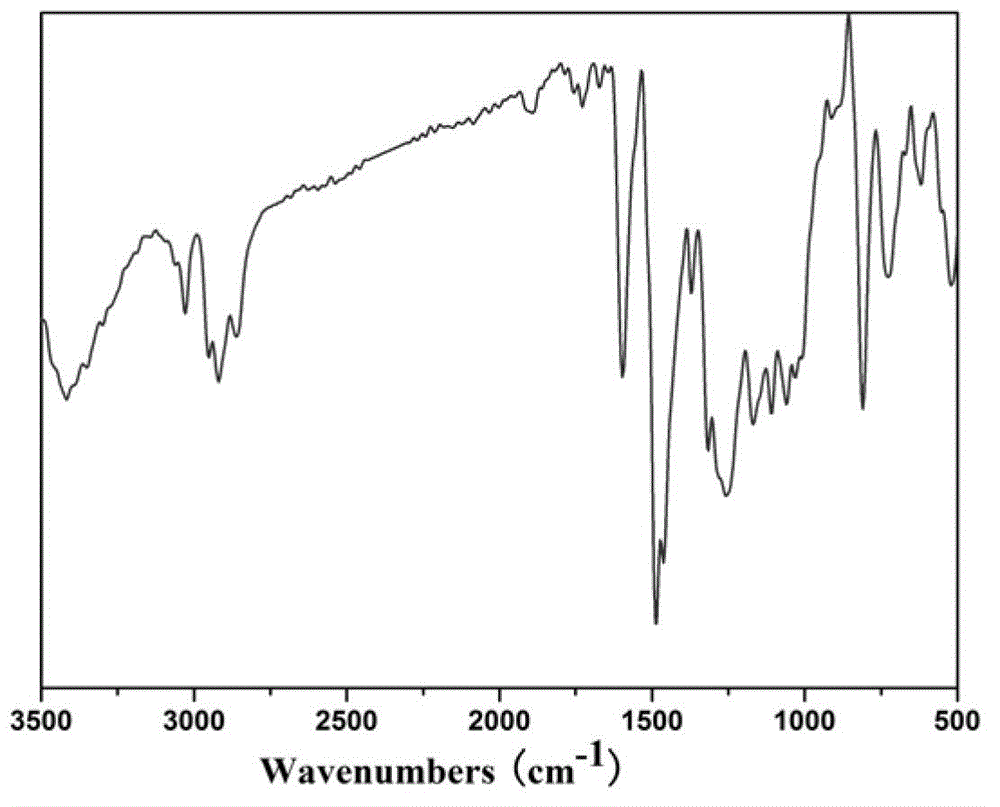
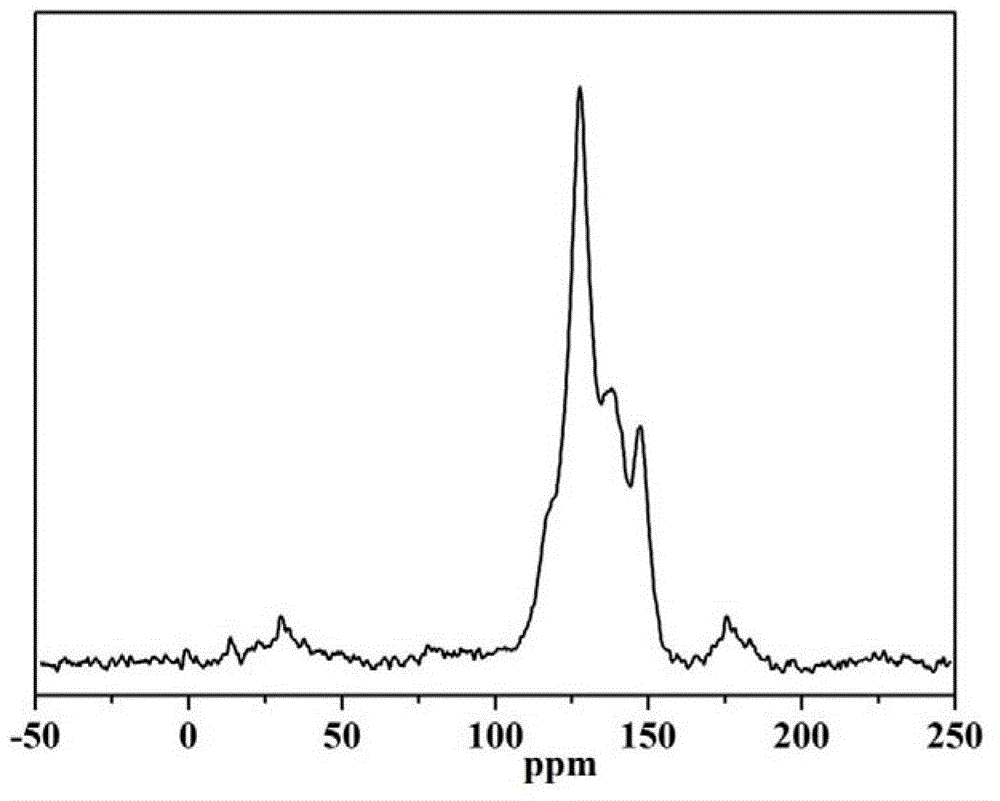
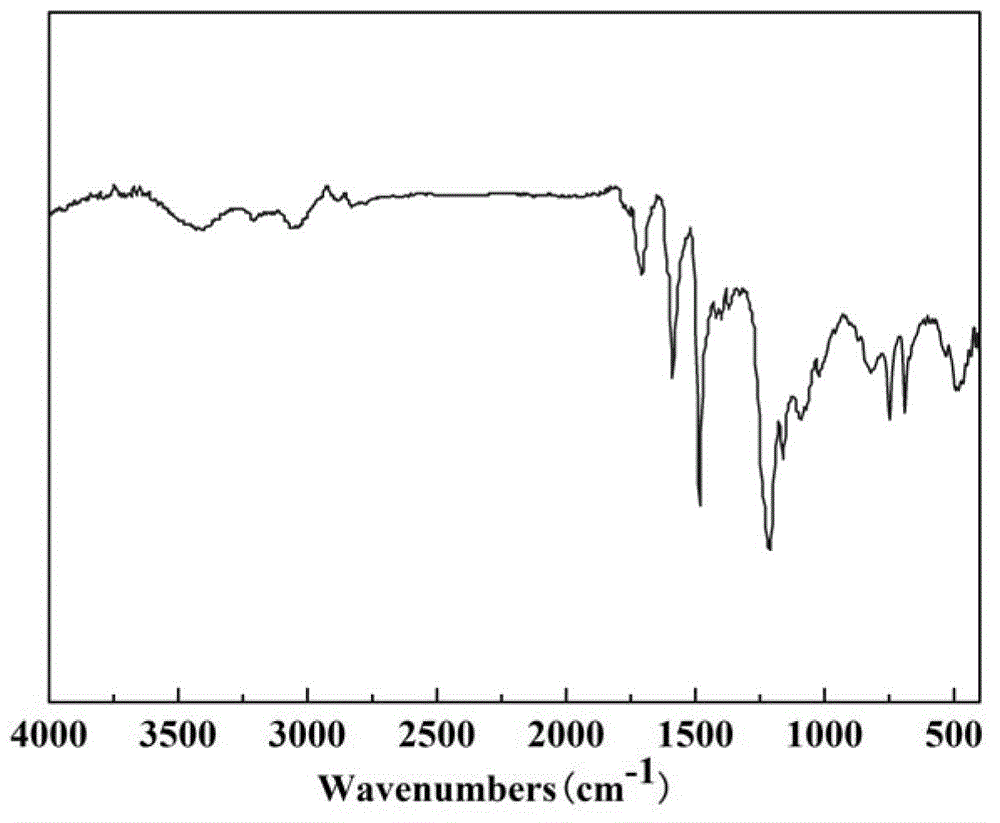
1,3,5-triazinyl nanopore organic aromatic heterocyclic polymer and preparation method thereof
A technology of polymers and nanopores, applied in separation methods, chemical instruments and methods, educts, etc., can solve problems such as difficult to achieve practical application and low adsorption capacity, achieve good adsorption performance and selective adsorption performance, and be widely used foreground effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Add and mix 0.2 parts of 2,4,6-trichloro-1,3,5-triazine and 0.2 parts of triphenylamine in molar ratio. The reaction uses chlorobenzene and nitrobenzene as a mixed solvent, and the entire reaction system is protected by argon. . Under the condition of stirring, add the methanesulfonic acid in the constant pressure dropping funnel dropwise to the reaction system at room temperature for 10 minutes, raise the temperature to 100°C, react for 10h, then cool down to room temperature, add methanesulfonic acid The amount of acid is 15 parts. The above mixed system was directly filtered to obtain a crude polymer product, and then washed with water, ethanol, tetrahydrofuran, acetone, chloroform, and acetone in sequence to obtain a polymer; vacuum-dried at 100°C for 3 days to obtain a polymer powder product.
[0039] The 5% thermal weight loss decomposition temperature of the polymer is 450°C, and the specific surface area reaches 862m 2 / g, the average pore size distribution is...
Embodiment 2
[0043]Add and mix 0.3 parts of 2,4,6-trichloro-1,3,5-triazine and 0.3 parts of tris-(pentaphenyl-tetra-)amine in molar ratio, and react with nitrobenzene as solvent, and the whole reaction The system is protected by nitrogen gas. Under the condition of stirring, anhydrous aluminum chloride is added into the reaction system at room temperature, and the aluminum chloride is added in three batches. The temperature of the system was raised to 50°C, and the reaction lasted for 6 hours, and then it was lowered to room temperature, and the amount of anhydrous aluminum chloride added was 8 parts. The above mixed system was directly filtered to obtain a crude polymer product, and then washed with water, acetone, chloroform, and acetone in sequence to obtain a polymer; vacuum-dried at 130°C for 1 day to obtain a polymer powder product.
[0044] The 5% thermal weight loss decomposition temperature of the polymer is 490°C, and the specific surface area reaches 1010m 2 / g, the average po...
Embodiment 3
[0047] Add and mix 0.4 part of 2,4,6-trichloro-1,3,5-triazine and 0.3 part of tetraphenylmethane in molar ratio, react with bromobenzene as solvent, and protect the whole reaction system with argon. Under the condition of stirring, trifluoromethanesulfonic acid was added dropwise to the reaction system at room temperature, trifluoromethanesulfonic acid was added dropwise at 0°C for 30 minutes, the temperature was raised to 25°C, and the reaction lasted for 24h. The amount of sulfonic acid is 5 parts. The above mixed system was directly filtered to obtain a crude polymer product, and then washed with water, tetrahydrofuran, chloroform, and acetone in sequence to obtain a polymer; vacuum-dried at 100°C for 2 days to obtain a polymer powder product.
[0048] The 5% thermal weight loss decomposition temperature of the polymer is 280°C, and the specific surface area reaches 750m 2 / g, the average pore size distribution is 3nm, in the low pressure area (P / P 0 =10 -5 ~10 -2 ), CO...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com